2-羟乙基二硫化物 | 1892-29-1
中文名称
2-羟乙基二硫化物
中文别名
2,2’-二硫二乙醇;双(2-羟乙基)二硫化物;双(2-羟乙基)二硫代;2-羟乙基二硫;2,2'-二硫二乙醇;双(2-羟基乙基)二硫醚;二硫代二乙二醇;2,2"-二硫二乙醇
英文名称
bis(2-hydroxyethyl) disulfide
英文别名
2,2'-dithiobisethanol;2-hydroxyethyl disulfide;2,2'-dithiodiethanol;2,2′-dithiodiethanol;2,2'-disulfanediylbis(ethan-1-ol);2,2′-disulfanediylbis(ethan-1-ol);2,2 ‘-dithiodithioethanol;di(2-hydroxyethyl) disulfide;2,2'-disulfanediyldiethanol;hydroxyethyl disulfide;2-(2-hydroxyethyldisulfanyl)ethanol
CAS
1892-29-1
化学式
C4H10O2S2
mdl
MFCD00002906
分子量
154.254
InChiKey
KYNFOMQIXZUKRK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25-27 °C (lit.)
-
沸点:158-163 °C/3.5 mmHg (lit.)
-
密度:1.261 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
最大波长(λmax):247nm(H2O)(lit.)
-
LogP:-0.3 at 19.9℃
-
物理描述:Liquid
-
保留指数:1425
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,没有已知危险反应,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:8
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:91.1
-
氢给体数:2
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S26,S39,S45
-
危险类别码:R23/24/25
-
WGK Germany:3
-
海关编码:2930909090
-
危险品运输编号:UN 2811 6.1/PG 3
-
危险类别:6.1
-
RTECS号:KK7525000
-
包装等级:III
-
危险标志:GHS05,GHS06
-
危险性描述:H301,H318
-
危险性防范说明:P280,P301 + P310,P305 + P351 + P338
-
储存条件:请将贮藏器密封保存,并存放在阴凉干燥处。确保工作环境具有良好的通风或排气设施。
SDS
| Name: | 2-Hydroxyethyl disulfide tech. 90% Material Safety Data Sheet |
| Synonym: | 2,2'-Dithiodiethano |
| CAS: | 1892-29-1 |
Synonym:2,2'-Dithiodiethano
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1892-29-1 | 2-Hydroxyethyl disulfide, tech. | 90% | 217-576-6 |
Risk Phrases: 23/24/25 41
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed. Risk of serious damage to eyes.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. Risk of serious damage to eyes.
Skin:
May cause skin irritation. Toxic in contact with skin.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. Toxic if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Toxic if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Wash mouth out with water. If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1892-29-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid or liquid
Color: after melting, clear yellow
Odor: stench
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 158 - 163 deg C @3.5mmHg
Freezing/Melting Point: 25 - 27 deg C
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Soluble.
Specific Gravity/Density: 1.288
Molecular Formula: C4H10O2S2
Molecular Weight: 154.25
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable.
Conditions to Avoid:
Excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, acid chlorides, acid anhydrides.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1892-29-1: KK7525000 LD50/LC50:
CAS# 1892-29-1: Oral, rat: LD50 = 173 mg/kg.
Carcinogenicity:
2-Hydroxyethyl disulfide, tech. - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLIDS, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLIDS, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLIDS, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
R 41 Risk of serious damage to eyes.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 38 In case of insufficient ventilation, wear
suitable respiratory equipment.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1892-29-1: No information available.
Canada
CAS# 1892-29-1 is listed on Canada's NDSL List.
CAS# 1892-29-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1892-29-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
2-羟乙基二硫化物是一种有机中间体,可通过3-巯基-1-丙醇与碘化钠和双氧水反应制备。该化合物可用于合成聚氨酯薄膜。
制备将0.92g(1mmol)的3-巯基-1-丙醇、1.5mg(0.01mmol)的碘化钠放入三口烧瓶中,加入3mL乙酸乙酯作为溶剂。在磁力搅拌下,缓慢滴加30%双氧水(0.11mL,1mmol)。滴加完毕后,在30℃下继续搅拌半小时。随后,加入15mL饱和硫代硫酸钠水溶液,并用15mL乙酸乙酯进行萃取。再使用15mL饱和食盐水洗涤,然后用无水硫酸钠干燥。最后通过旋蒸除去溶剂,得到2-羟乙基二硫化物,产率高达98%。
检测方法采用有机溶剂和水的混合溶液作为流动相进行洗脱,分离出2-羟乙基二硫化物及其杂质(包括左亚叶酸钙的相关物质)。所述相关物质为左亚叶酸钙的杂质。流动相流速设定在0.9~1.1ml/min之间;使用检测波长247nm,并通过外标法定量分析该化合物含量,确保准确度。乙腈与水的比例为1:9;色谱柱温度控制在25~35℃范围内。此方法专属性强、耐用性好,能够有效快速地测定杂质含量。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-hydroxyethyl ethyldisulfide —— C4H10OS2 138.255 —— 3,4-dithiapentan-1-ol 85351-54-8 C3H8OS2 124.228 —— bis(2-methoxyethyl) disulfide 69177-66-8 C6H14O2S2 182.308 —— 2-((2-aminoethyl)dithio)-1-ethanol 15579-01-8 C4H11NOS2 153.269 —— 2-((2-bromoethyl)disulfanyl)ethanol 113398-38-2 C4H9BrOS2 217.151
反应信息
-
作为反应物:描述:参考文献:名称:Mass Spectral Studies on Vinylic Degradation Products of Sulfur Mustards under Gas Chromatography/Mass Spectrometry Conditions摘要:硫芥子是一类气泡剂化学战剂,它们在环境样品中迅速降解。硫芥子最可行的降解产物是氯乙基乙烯化合物和二乙烯化合物,它们分别通过从硫芥子中消除一个和两个HCl分子形成。在环境样品中检测和表征这些降解产物对于验证硫芥子的使用是重要的证据。在这项研究中,我们合成了一组硫芥子降解产物,即二乙烯化合物(1-7)和氯乙基乙烯化合物(8-14),并使用气相色谱/质谱联用(GC/MS)在电子电离(EI)和化学电离(CI)(甲烷)条件下进行表征。研究化合物的EI质谱主要包括由于有或无氢迁移而导致的自由裂解的碎片离子。二乙烯化合物(1-7)显示[M - SH]+离子,而氯乙基乙烯化合物(8-14)显示[M - Cl]+和[M - CH2CH2Cl]+离子。甲烷/CI质谱显示[M + H]+离子并提供分子量信息。还计算了研究化合物的GC保留指数(RI)值。EI和CI质谱数据以及RI值对于离线分析以验证化学武器公约以及参加官方禁化武组织的熟练测试非常有用。DOI:10.1255/ejms.1398
-
作为产物:参考文献:名称:LINAC / LASER测定巯基乙醇,半胱胺和N-乙酰基-1-半胱氨酸水溶液中巯基与巯基的巯基和羟自由基反应的绝对速率常数†摘要:合并的脉冲辐解/光解激光的技术已被用于研究巯基乙醇,半胱胺,以及二硫化自由基阴离子形成反应Ñ乙酰基升-半胱氨酸在7.0-13.0的pH范围内。巯基的一次电子氧化形成的瞬态自由基阴离子的光解扰动了二硫键自由基阴离子/噻吩基自由基的平衡,从而可以从吸收漂白和随后的回收中唯一确定巯基自由基与母体巯基反应的速率常数。 。将这些依赖于pH的值与测得的二硫自由基阴离子平衡常数结合起来,以计算一阶二硫自由基阴离子解离速率常数。通过计算机模拟已建立的二硫键自由基生长机理的模型,计算了羟基自由基与各个巯基物质反应的速率常数。将这些值与先前报道的通过竞争动力学确定的值进行对比。DOI:10.1021/jp953067v
-
作为试剂:描述:苯-1,3,5-三甲基硫醇 在 2-羟乙基二硫化物 、 tetramethylquanidine 作用下, 以 氘代甲醇 、 氘代苯 为溶剂, 生成 alkaline earth salt of/the/ methylsulfuric acid参考文献:名称:硫醇-二硫化物交换的结构-反应性关系摘要:平衡常数由 36 个二硫醇和三硫醇与衍生自 2-巯基乙醇或二硫苏糖醇的二硫化物之间的硫醇-二硫化物交换确定。反应在甲醇-d/水性缓冲液 (pH 7) 或甲醇中进行,在 25°C 下,使用 NMR 光谱跟踪反应。这些数据用于根据还原电位对二硫醇进行排序并推断其结构氧化时由它们形成的二硫化物。二硫醇的还原能力与氧化时形成的含二硫化物环的大小之间存在普遍的相关性:形成六元环的二硫醇还原性最强(K = 103-105 M 相对于氧化的 2-巯基乙醇);五元和七元环的还原性降低约 1 个数量级。类似于 1 的化合物,2-乙二硫醇在相对稀释的溶液 (- 1 mM) 中形成环状双(二硫化物)二聚体,但在较高浓度下聚合。其他类别的二硫醇在氧化时形成聚合物。DOI:10.1021/ja00256a040
文献信息
-
Reactions of cysteine sulfenyl thiocyanate with thiols to give unsymmetrical disulfides作者:Susan L. Alguindigue Nimmo、Kelemu Lemma、Michael T. AshbyDOI:10.1002/hc.20340日期:2007.7Cysteine sulfenyl thiocyanate (CSSCN) reacts with thiols at pH 0 to cleanly yield disulfides. 2-Mercaptoethanol (2-MESH), 3-mercaptopropionic acid (3-MPASH), penicillamine (PENSH), and glutathione (GSH) react with CSSCN to give the corresponding mixed disulfides: 2-MESSC, 3-MPASSC, PENSSC, and GSSC. These compounds are stable at pH 0 and have been characterized by 1H and 13C NMR spectroscopy. © 2007半胱氨酸硫氰酸亚硫基酯 (CSSCN) 在 pH 值为 0 时与硫醇反应,干净地生成二硫化物。2-巯基乙醇 (2-MESH)、3-巯基丙酸 (3-MPASH)、青霉胺 (PENSH) 和谷胱甘肽 (GSH) 与 CSSCN 反应生成相应的混合二硫化物:2-MESSC、3-MPASSC、PENSSC 和GSSC。这些化合物在 pH 值为 0 时稳定,并已通过 1H 和 13C NMR 光谱进行表征。© 2007 Wiley Periodicals, Inc. 18:467–471, 2007;在线发表于 Wiley InterScience (www.interscience.wiley.com)。DOI 10.1002/hc.20340
-
Polymer-Supported Diaryl Selenoxide and Telluroxide as Mild and Selective Oxidizing Agents作者:Nan Xing Hu、Yoshio Aso、Tetsuo Otsubo、Fumio OguraDOI:10.1246/bcsj.59.879日期:1986.3Polystyrene-bound diaryl selenoxide and telluroxide have been prepared, which behaved as mild oxidizing agents for thiols to disulfides, phosphines to phosphine oxides, hydroquinone and catechol to p- and o-benzoquinones, and thioketones to oxo compounds. The telluroxide completed these reactions in shorter periods or under milder conditions than the selenoxide. In addition, they effected novel solvent-dependent
-
Combination of FBPase inhibitors and insulin sensitizers for the treatment of diabetes申请人:Metabasis Therapeutics, Inc.公开号:US06756360B1公开(公告)日:2004-06-29Pharmaceutical compositions containing an FBPase inhibitor and an insulin sensitizer are provided as well as methods for treating diabetes and diseases responding to increased glycemic control, an improvement in insulin sensitivity, a reduction in insulin levels, or an enhancement of insulin secretion.
-
SYNTHESIS OF MORPHOLINO OLIGOMERS USING DOUBLY PROTECTED GUANINE MORPHOLINO SUBUNITS申请人:REEVES MATTHEW DALE公开号:US20090131624A1公开(公告)日:2009-05-21Morpholino compounds are provided having the structure: where R 1 is selected from the group consisting of lower alkyl, di(lower alkyl)amino, and phenyl; R 2 is selected from the group consisting of lower alkyl, monocyclic arylmethyl, and monocyclic (aryloxy)methyl; R 3 is selected from the group consisting of triarylmethyl and hydrogen; and Y is selected from the group consisting of: a protected or unprotected hydroxyl or amino group; a chlorophosphoramidate group; and a phosphorodiamidate linkage to the ring nitrogen of a further morpholino compound or a morpholino oligomer. Such compounds include doubly protected morpholino guanine (MoG) monomers. Also described is their use in synthesis of morpholino oligomers.
-
PROGRAMMABLE THERMORESPONSIVE GELS申请人:City of Hope公开号:US20200121598A1公开(公告)日:2020-04-23Provided herein, inter alia, are non-crosslinked polymers that possess thermoresponsive properties. These polymers possess a cleavable bond that breaks under certain conditions The disclosure also provides pharmaceutical compositions containing the polymers and therapeutic agents, methods for delivering the therapeutic agents, and kits, syringes, and catheters containing the polymer compositions and therapeutic agents.本文提供了具有热响应性能的非交联聚合物。这些聚合物具有在特定条件下断裂的可切割键。该公开还提供了含有这些聚合物和治疗剂的药物组合物、传递治疗剂的方法,以及含有聚合物组合物和治疗剂的工具包、注射器和导管。
表征谱图
-
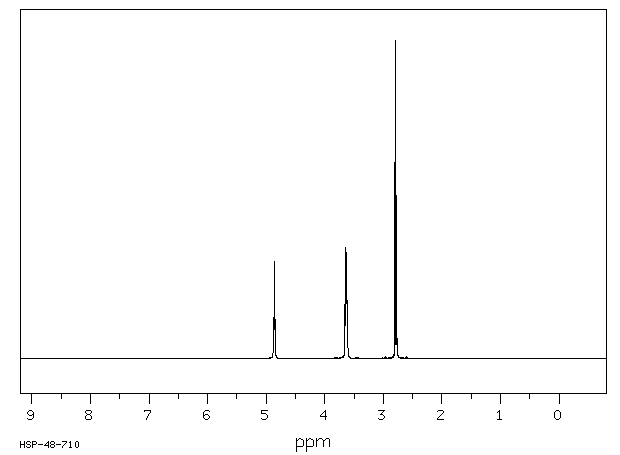
氢谱1HNMR
-
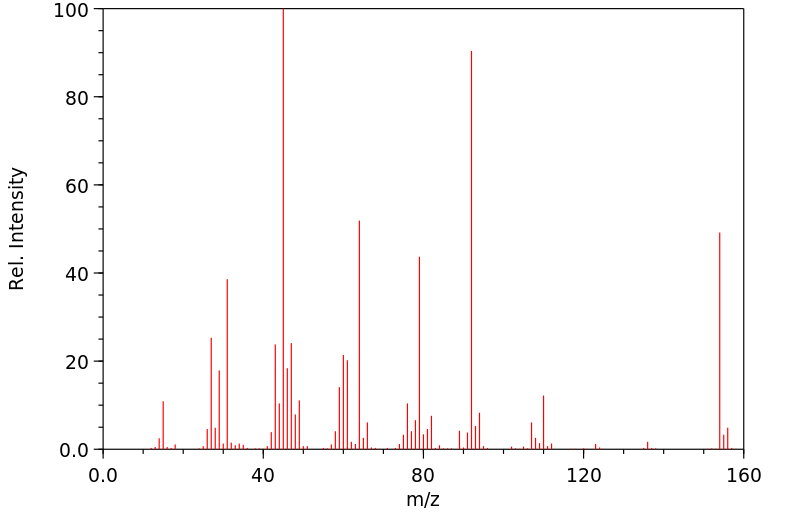
质谱MS
-
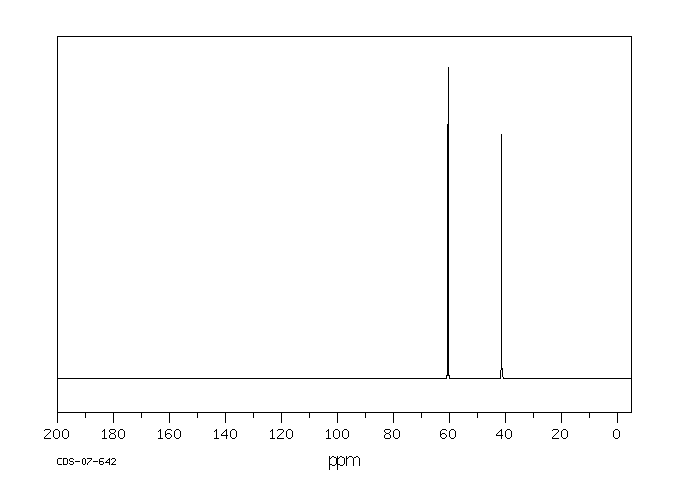
碳谱13CNMR
-
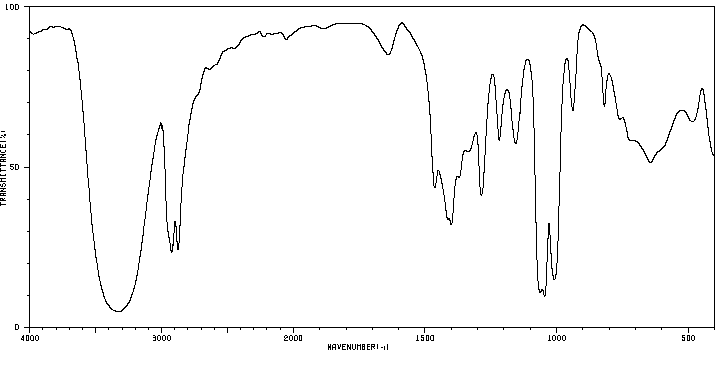
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高胱胺
胱胺
福多司坦杂质
甲基异戊基二硫醚
甲基异丙基二硫醚
甲基半胱胺
甲基丙基二硫醚
甲基丙-1-烯基二硫醚
甲基[2-甲基-1-(甲硫基)丁基]过硫化物
甲基3-甲基-1-丁烯基二硫醚
甲基-D6 二硫醚
氧化福美双
次氮基-氰基二硫基-甲烷
敌灭生
戊基甲基二硫醚
异丙基二硫醚
哌啶并,3-[2-(2-乙基苯基)肼基]-
叔丁基硫基二甲基氨基二硫代甲酸酯
叔丁基二硫
反式丙烯基丙基二硫
双羟甲基二硫化物
双正癸基二硫醚
双十六烷基二硫化物
双(十二烷基硫烷基硫代羰基)二硫化物
双(十三氟己基)二硫醚
双(三氟硫代乙酰基)二硫化物
双(2,2-二乙氧基乙基)二硫化物
双(2,2,2-三氟乙基)二硫化物
双(16-羟基十六烷基)二硫化物
双(11-羟基十一烷基)二硫化物
双(1,2-二甲基-2-氯丙基)二硫化物
原文:多(2,3-环硫烷基)二硫化物,但查不到猜测:双(2,3-环硫丙基)二硫化物
二黄原酸
二肉豆蔻基二硫醚
二硫氨磷汀
二硫化二正丁基黄原酸酯
二硫化二异丙基黄原酸酯
二硫化,二环辛基
二硫化,二(1-羰基十六烷基)
二硫代氨基甲酰二硫醚
二硫代戊酯
二甲基二硫
二甲基-13C2二硫
二环己基二硫化物
二环丙基二硫
二氯-[(甲基二硫烷基)甲氧基]甲烷
二氯-(甲基二硫烷基)甲烷
二正辛基二硫
二正庚基二硫醚
二正壬基二硫醚