1-(4-甲氧基苯基)-1-环戊烷甲酸 | 43050-28-8
物质功能分类
中文名称
1-(4-甲氧基苯基)-1-环戊烷甲酸
中文别名
1-(4-甲氧基苯基)环戊烷羧酸;1-(4-甲氧基苯基)-1-环戊烷羧酸
英文名称
1-(4-methoxyphenyl)cyclopentanecarboxylic acid
英文别名
1-(4-methoxyphenyl)cyclopentane-1-carboxylic acid
CAS
43050-28-8
化学式
C13H16O3
mdl
MFCD00001374
分子量
220.268
InChiKey
OMMROWIAJMZSLF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:151-156 °C
-
沸点:321.2°C (rough estimate)
-
密度:1.1101 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.461
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S24/25
-
海关编码:2918990090
-
储存条件:2-8℃
SDS
| Name: | 1-(4-Methoxyphenyl)-1-cyclopentanecarboxylic acid 95% Material Safety Data Sheet |
| Synonym: | 1-(4-Methoxyphenyl)cyclopentane-1-carboxylic aci |
| CAS: | 43050-28-8 |
Synonym:1-(4-Methoxyphenyl)cyclopentane-1-carboxylic aci
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 43050-28-8 | 1-(4-Methoxyphenyl)-1-cyclopentanecarb | 95 | 256-063-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 43050-28-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light brown
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 151.00 - 153.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C13H16O3
Molecular Weight: 220.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 43050-28-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(4-Methoxyphenyl)-1-cyclopentanecarboxylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 43050-28-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 43050-28-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 43050-28-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-Methoxyphenyl-1-cyclopentancarbaldehyd 43050-26-6 C13H16O2 204.269 1-(4-甲氧基苯基)-1-环戊腈 1-(p-Methoxyphenyl)cyclopentanecarbonitrile 1206-15-1 C13H15NO 201.268 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(4-羟基-苯基)环戊烷甲酸 1-(4-hydroxyphenyl)cyclopentanecarboxylic acid 91496-64-9 C12H14O3 206.241 [1-(4-甲氧基苯基)环戊基]甲醇 <1-(p-Methoxyphenyl)cyclopentyl>carbinol 95266-26-5 C13H18O2 206.285 —— 1-(4-methoxyphenyl)cyclopentanecarbonyl chloride 63200-97-5 C13H15ClO2 238.714 —— N-methoxy-1-(4-methoxyphenyl)cyclopentane-1-carboxamide 1072809-94-9 C14H19NO3 249.31 —— <1-(p-Methoxyphenyl)cyclopentyl>carbinyl tosylate 162780-90-7 C20H24O4S 360.474
反应信息
-
作为反应物:描述:1-(4-甲氧基苯基)-1-环戊烷甲酸 在 氯化亚砜 、 三乙胺 作用下, 以 甲苯 为溶剂, 反应 4.0h, 生成 2-<2-(diethylamino)ethoxy>ethyl 1-(4-methoxyphenyl)-1-cyclopentanecarboxylate参考文献:名称:新型的1-苯基环烷羧酸衍生物是有效的选择性σ1配体。摘要:Carbetapentane(1-苯基-1-环戊烷羧酸1,2- [2-(2-(二乙基氨基)乙氧基]乙基]乙酯与sigma位高结合,是一种有效的镇咳药,抗惊厥药和痉挛药。然而,Carbetapentane也在毒蕈碱结合位点相互作用,目前尚不清楚这些受体系统中的任何一个是否参与了该药物的作用机制。为了确定这些精神活动是否可以归因于sigma位点的相互作用,制备了一系列的Carbetapentane类似物。苯环取代;收缩,膨胀和用环戊基环的甲基取代;用酰胺,甲醚和甲胺代替羧酸酯官能团;并且研究了用吗啉代或哌啶子基部分取代N,N-二乙基取代基。评估了所有这些新的类似物与sigma 1和sigma 2位点的结合,并比较了毒蕈碱m1和m2与PCP(1-(1-苯基环己基)哌啶)受体的结合。所有化合物对sigma 1的选择性都高于sigma 2的位置,三个选择性最高的类似物是化合物34(65倍),35(7DOI:10.1021/jm00041a006
-
作为产物:描述:参考文献:名称:Direct Lactonization of 2-Arylacetic Acids through Pd(II)-Catalyzed C–H Activation/C–O Formation摘要:Palladium-catalyzed direct lactonization of 2-arylacetic acids through a reaction sequence that includes C-H activation/C-O formation is reported. This method provides a concise and efficient pathway to synthesize fully functionalized benzofuranone derivatives, which are highly relevant to bioactive natural and synthetic products.DOI:10.1021/ol303569b
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] MODULATORS OF ATP-BINDING CASSETTE TRANSPORTERS<br/>[FR] MODULATEURS DE TRANSPORTEURS DE TYPE CASSETTE DE LIAISON A L'ATP申请人:VERTEX PHARMA公开号:WO2005075435A1公开(公告)日:2005-08-18The present invention relates to modulators of ATP-Binding Cassette ('ABC') transporters or fragments thereof, including Cystic Fibrosis Transmembrane Conductance Regulator ('CFTR'), compositions thereof, and methods therewith. The present invention also relates to methods of treating ABC transporter mediated diseases using such modulators (I) or a pharmaceutically acceptable salt thereof, wherein: Ht is a 5-membered heteroaromatic ring containing 1-4 heteroatoms selected from O, S, N or NH, wherein said ring is optionally fused to a 6-membered monocyclic or 10-membered bicyclic carbocyclic or heterocyclic, aromatic or non-aromatic ring, wherein Ht is optionally substituted with w occurrences of -WRw, wherein w is 0-5; ring A is 3-7 membered monocyclic ring having 0-3 heteroatoms selected from O, S, N, or NH, wherein ring A is optionally substituted with q occurrences of QRQ; ring B is optionally fused to 5-6 membered carbocyclic or heterocyclic, aromatic or non-aromatic ring .本发明涉及ATP结合盒('ABC')转运蛋白或其片段的调节剂,包括囊性纤维化跨膜传导调节蛋白('CFTR'),以及相关的组合物和方法。本发明还涉及使用这些调节剂(I)或其药学上可接受的盐治疗ABC转运蛋白介导的疾病的方法,其中:Ht是含有1-4个来自O、S、N或NH的杂原子的5元杂芳环,其中所述环可选择地与含有0-5个-WRw的w出现的6元单环或10元双环碳环或杂环、芳香或非芳香环融合;环A是具有0-3个来自O、S、N或NH的杂原子的3-7元单环,其中环A可选择地与q出现的QRQ取代;环B可选择地与5-6元碳环或杂环、芳香或非芳香环融合。
-
Modulators of ATP-binding cassette transporters申请人:Hadida Ruah S. Sara公开号:US20070244159A1公开(公告)日:2007-10-18Compounds of the present invention and pharmaceutically acceptable compositions thereof, are useful as modulators of ATP-Binding Cassette (“ABC”) transporters or fragments thereof, including Cystic Fibrosis Transmembrane Conductance Regulator (“CFTR”). The present invention also relates to methods of treating ABC transporter mediated diseases using compounds of the present invention.本发明的化合物及其药学上可接受的组合物,可用作调节ATP结合盒(“ABC”)转运蛋白或其片段的工具,包括囊性纤维化跨膜传导调节蛋白(“CFTR”)。本发明还涉及使用本发明的化合物治疗ABC转运蛋白介导的疾病的方法。
-
MODULATORS OF CYSTIC FIBROSIS TRANSMEMBRANE CONDUCTANCE REGULATOR PROTEIN申请人:AbbVie S.à.r.l.公开号:US20170305891A1公开(公告)日:2017-10-26The present invention provides for compounds of formula (I) wherein R 1 , m, Z, G 1 , R 2 , and R 3 have any of the values defined in the specification, and pharmaceutically acceptable salts thereof, that are useful as agents in the treatment of diseases and conditions mediated and modulated by CFTR, including cystic fibrosis, Sjögren's syndrome, pancreatic insufficiency, chronic obstructive lung disease, and chronic obstructive airway disease. Also provided are pharmaceutical compositions comprised of one or more compounds of formula (I).本发明提供了以下式(I)的化合物,其中R1、m、Z、G1、R2和R3具有规范中定义的任何值,以及其药学上可接受的盐,这些化合物可用作治疗由CFTR介导和调节的疾病和症状的药物,包括囊性纤维化、Sjögren综合征、胰腺功能不全、慢性阻塞性肺病和慢性阻塞性气道疾病的药物。还提供了由一个或多个式(I)化合物组成的药物组合物。
表征谱图
-
氢谱1HNMR
-
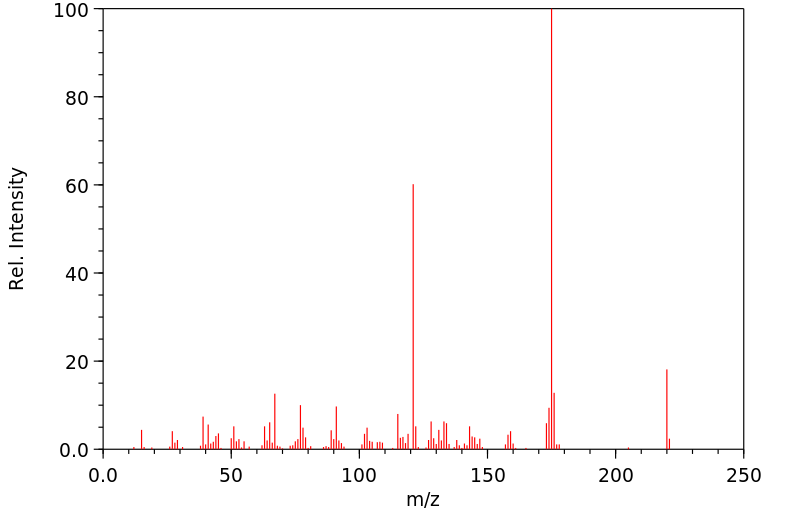
质谱MS
-
碳谱13CNMR
-
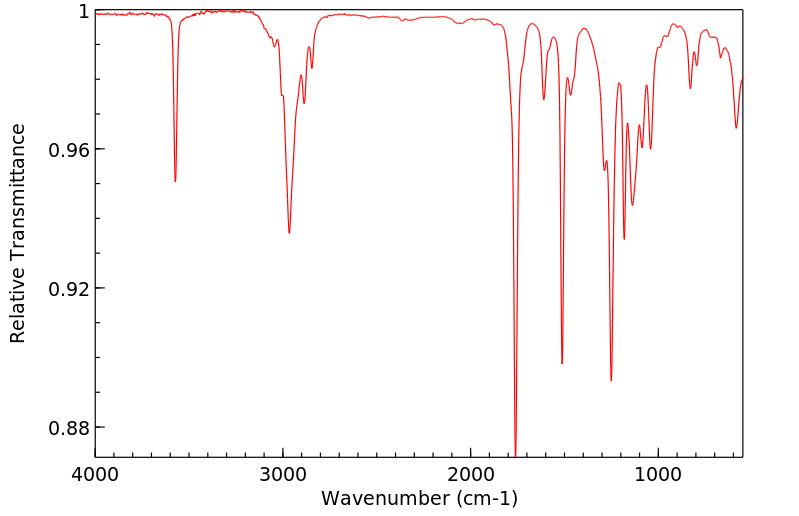
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯