N-硝二甲胺 | 4164-28-7
分子结构分类
中文名称
N-硝二甲胺
中文别名
——
英文名称
N-nitrodimethylamine
英文别名
N,N-dimethylnitramine;dimethylnitramine;N,N-dimethylnitramide
CAS
4164-28-7
化学式
C2H6N2O2
mdl
MFCD00626512
分子量
90.0818
InChiKey
XRWKADIRZXTTLH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:58°C
-
沸点:167.26°C (rough estimate)
-
密度:1.1090
-
蒸汽压力:0.36 mmHg
-
大气OH速率常数:3.84e-12 cm3/molecule*sec
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:6
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:49.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2928000090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Mechanisms of Nitramine Thermolysis摘要:The thermal decomposition of a number of nitramines was studied in dilute solution and in the melt, The nitramines included acyclic mononitramines [dimethylnitramine (DMN), diethylnitramine (DEN), dipropylnitramine (DPN), and diisopropylnitramine (DIPN)], cyclic mononitramines [N-nitropiperidine (NPIP) and N-nitropyrrolidine (NPyr)], cyclic dinitramines [N-dinitropiperazine (pDNP), 1,3-dinitro-1,3-diazacyclopentane (DNI), and 1,3-dinitro-1,3-diazacyclohexane (mDNP)], and 1,3,5-trinitro-1,3,5-triazocyclohexane (RDX), octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), hexanitrohexaazaisowurtzitane (HNIW), and 1,3,3-trinitroazetidine (TNAZ). For the acyclic and cyclic mono- and dinitramines, the corresponding nitrosamines were the only or major condensed-phase product. Kinetics and activation parameters were determined for the thermolysis of dilute solutions (0.01-1.0 wt %) over the range 200-300 degrees C. The thermolyses were found to be first-order with the rate constants unaffected by the use of deuterated solvent. As the nitramines became more complex than dimethylnitramine (DMN), the rate of decomposition increased and the product distribution became more complex. As the length of the aliphatic chain increased (DMN < DEN < DPN), the rate of thermolysis increased, yet nitrosamine remained the only observed condensed-phase product. When a secondary carbon was attached to the N-nitramine (DIPN) rather than the primary (DPN), the rate of decomposition increased and a new condensed-phase product was observed. Among the cyclic nitramines, the rate of decomposition increased as the number of NNO2 groups increased (NPIP < pDNP; NPyr < DNI; mDMP < RDX). The position of the nitramine groups affected the decomposition: meta NNO2 groups (mDNP) decomposed faster than para (pDNP). Ring strain decreased stability: mDNP < DNI; HMX < RDX. In complex nitramines, the increase in decomposition rate, the appearance of new products, and the change in the relative importance of nitrosamine and of N-2 and N2O are attributed to new decomposition routes available to them. However, since complex nitramines (e.g. RDX) maintain first-order kinetics and since most have activation energies in the range of 40-50 kcal/mol, it is believed that the triggering mechanism remains N-NO2 homolysis. Intramolecular hydrogen transfer is also considered an important mode of nitramine decomposition.DOI:10.1021/j100079a019
-
作为产物:参考文献:名称:气相中二甲基亚硝胺的光解†摘要:在室温下辐照到S 1(nπ*)S 0(363.5 nm)和S 2(ππ*)S 0(248.1 nm)跃迁后,已经研究了气相(〜1 Torr)中二甲基亚硝胺的光分解。具有单位的量子产率,激励成S 1状态产生的片段(CH 3)2 N·和NO,然后重新结合不留光化。O 2的添加仅产生一种光产物,即(CH 3)2 NNO 2。辐射到S 2状态,生成的产物CH 2 NCH 3(通过毛细管气相色谱质谱法鉴定了2 NCH 3)3,CH 2 NOH,N 2 O,NO,H 2和N 2。在存在N 2作为缓冲气体的情况下,光产物仅为CH 2 NCH 3,(CH 2 NCH 3)3,N 2 O和H 2。对于这两种激发条件,提出了一种机制,该机制涉及裂解N,N键作为主要的主要光解过程。DOI:10.1002/hlca.19810640405
-
作为试剂:描述:endo-Dicyclopentadien 在 盐酸 、 lithium aluminium tetrahydride 、 N-硝二甲胺 、 氧气 作用下, 生成 exo-9-dimethylamino-endo-tricyclo<5.2.1.0>dec-3-en-exo-8-ol 、 trans,cic,trans-2,4-bishydroxymethylbicyclo<3.3.0>oct-6-ene参考文献:名称:Chow, Yuan L.; Richard, Herve, Journal of the Chemical Society. Perkin transactions I, 1982, p. 1405 - 1418摘要:DOI:
文献信息
-
The nitrolysis of N,N-dialkylcarboxamides in liquid carbon dioxide作者:I. V. Kuchurov、I. V. Fomenkov、S. G. ZlotinDOI:10.1007/s11172-010-0371-1日期:2010.11A method for the synthesis of N,N-dialkylnitramines by the ninrolysis of N,N-dialkylcarboxamides with a mixture of N2O5 and 100% HNO3 in liquid carbon dioxide was developed.
-
Synthesis of N,N-dialkylnitramines from secondary ammonium nitrates in liquid or supercritical carbon dioxide作者:I. V. Kuchurov、I. V. Fomenkov、S. G. ZlotinDOI:10.1007/s11172-009-0282-1日期:2009.10An efficient explosion-proof method was developed for the preparation of N,N-dialkylnitramines by treatment of dialkylammonium nitrates with a mixture of nitric acid and acetic anhydride in the presence of ZnCl2 in liduid or supercritical carbon dioxide.
-
Formation of <i>N</i>-Nitrosamines and <i>N</i>-Nitramines by the Reaction of Secondary Amines with Peroxynitrite and Other Reactive Nitrogen Species: Comparison with Nitrotyrosine Formation作者:Mitsuharu Masuda、Howard F. Mower、Brigitte Pignatelli、Irena Celan、Marlin D. Friesen、Hoyoku Nishino、Hiroshi OhshimaDOI:10.1021/tx990120o日期:2000.4.1mechanism, involving one-electron oxidation by peroxynitrite of secondary amines to form amino radicals (R(2)N(*)), which react with nitric oxide ((*)NO) or nitrogen dioxide ((*)NO(2)) to yield nitroso and nitro secondary amines, respectively. Reaction of morpholine with NO(*) and superoxide anion (O(2)(*)(-)), which were concomitantly produced from spermine NONOate and by the xanthine oxidase systems, respectively活性氮物质包括氮氧化物(N(2)O(3)和N(2)O(4)),过氧亚硝酸盐(ONOO(-))和硝酰氯(NO(2)Cl)被暗示为引起炎症和癌症的原因。我们研究了仲胺与过氧亚硝酸盐的反应,发现同时形成了N-亚硝胺和N-硝胺。在碱性pH下,过氧亚硝酸盐比在中性pH下更容易被亚硝酸盐亚硝化,而在pH 8.5下,过氧亚硝酸盐的硝化作用是最佳的。该反应中亚硝基吗啉的收率是碱性pH下亚硝基吗啉的3倍,而在pH <7.5下形成的亚硝基吗啉是亚硝基吗啉的2倍。对于吗啉-过亚硝酸盐反应,低浓度的碳酸氢盐可增强硝化作用,但过量的碳酸氢盐可抑制硝化作用。亚硝酸盐被过量的碳酸氢盐抑制。在此基础上,我们提出了一种自由基机理,涉及一种通过仲胺的过氧亚硝酸盐单电子氧化形成氨基(R(2)N(*))的基团,该基团与一氧化氮((*)NO)或二氧化氮反应((*)NO(2))分别产生亚硝基和硝基仲胺。吗啉与NO(*)和超氧阴离子(
-
Clean nitrations: Novel syntheses of nitramines and nitrate esters by nitrodesilylation reactions using dinitrogen pentoxide (N 2 O 5 )作者:Ross W. Millar、Simon P. PhilbinDOI:10.1016/s0040-4020(97)00093-8日期:1997.3with conventional substrates (amines or alcohols). These nitrodesilylation reactions proceed cleanly and in good yield, and the scope of the reaction is illustrated by. 29 examples, some of which produce high energy compounds, notably plasticisers and an energetic polymer precursor. These reactions are therefore potentially clean nitrations for the manufacture of energetic compounds which will minimise
-
Electroreduction of primary nitramines on platinum in MeCN作者:V. A. Petrosyan、V. A. Frolovsky、D. A. Sad9lenkoDOI:10.1007/bf02494405日期:1999.1Reduction of primary nitramines RNHNO2 (R=Me, Et, Pri, α-pyridyl) in anhydrous MeCN at a Pt cathode was studied by voltammetry and electrolysis. The process includes one-electron transfer and nitramine deprotonation to give the corresponding anions. The products ofN-alkylation of these anions can be obtained only when their potassium salts are used (but not tetrabutylammonium salts). This is due to
表征谱图
-
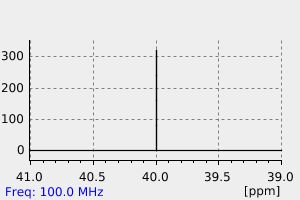
氢谱1HNMR
-
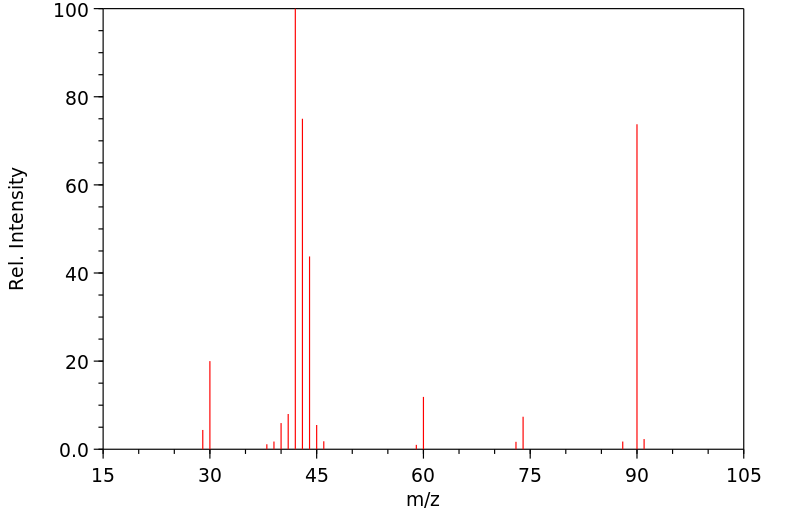
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-硝基环己基乙酸酯
顺式-2-硝基-6-甲基环己酮
雷尼替丁杂质18
铝硝基甲烷三氯化物
钾离子载体III
重氮(硝基)甲烷
醛基-七聚乙二醇-叠氮
过氧亚甲基
辛腈,4-氟-4-硝基-7-羰基-
辛烷,1,2-二氯-1-硝基-
赤霉素A4+7(GA4:GA7=65:35)
苄哒唑
羟胺-四聚乙二醇-叠氮
羟胺-三乙二醇-叠氮
米索硝唑
磷酸十二醇酯
碘硝基甲烷
碘化e1,1-二甲基-4-羰基-3,5-二(3-苯基-2-亚丙烯基)哌啶正离子
硝酰胺
硝基脲银(I)复合物
硝基甲醇
硝基甲烷-d3
硝基甲烷-13C,d3
硝基甲烷-13C
硝基甲烷-(15)N
硝基甲烷
硝基甲基甲醇胺
硝基环辛烷
硝基环戊烷
硝基环戊基阴离子
硝基环庚烷
硝基环己烷锂盐
硝基环己烷钾盐
硝基环己烷
硝基环丁烷
硝基氨基甲酸
硝基新戊烷
硝基二乙醇胺
硝基乙醛缩二甲醇
硝基乙醛缩二乙醇
硝基乙腈
硝基乙烷-D5
硝基乙烷-1,1-d2
硝基乙烷
硝基乙烯
硝基丙烷
硝基丙二醛(E,E)-二肟
硝基丙二腈
硝基-(3-硝基-[4]吡啶基)-胺
硝乙醛肟