呋喃 | 110-00-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-85.6 °C
-
沸点:67 °C10 mm Hg(lit.)
-
密度:0.936 g/mL at 25 °C(lit.)
-
蒸气密度:2.35 (vs air)
-
闪点:160 °F
-
溶解度:醇类:易溶
-
介电常数:3.0(25℃)
-
暴露限值:NIOSH: IDLH 13 ppm
-
LogP:1.34
-
物理描述:Furan appears as a clear colorless liquid with a strong odor. Flash point below 32°F. Less dense than water and insoluble in water. Vapors heavier than air.
-
颜色/状态:Colorless liquid, turns brown upon standing; color change is retarded if a small amount of water is added
-
气味:Ethereal
-
蒸汽密度:2.3 (EPA, 1998) (Relative to Air)
-
蒸汽压力:600 mm Hg at 25 °C /from experimentally derived coefficients/
-
大气OH速率常数:4.05e-11 cm3/molecule*sec
-
自燃温度:390 °C
-
分解:Furan is a heat-stable compound although, @ 670 °C in the absence of catalyst, or @ 360 °C in the presence of nickel, it decomposes to form a mixture consisting mainly of carbon monoxide, hydrogen, and hydrocarbons.
-
粘度:0.38 cP at 20 °C
-
燃烧热:-500.1 kg cal/mole (at constant volume)
-
汽化热:95.5 cal/g at 31.2 °C
-
折光率:Index of refraction: 1.4214 at 20 °C/D
-
保留指数:500;500;498;485;500;494.7;492;500;498
-
稳定性/保质期:
2-卤代呋喃、酰基呋喃、氰基呋喃及磺酰基呋喃的合成 作为富电芳香杂环,呋喃能与多种亲电试剂反应,以制备卤代呋喃、酰基呋喃、氰代呋喃及其他杂原子取代呋喃等产物(式1)。通常情况下,C-2位比C-3位更容易受到亲电试剂的进攻。
烷基呋喃的合成 烷基呋喃可通过呋喃进行亲电取代反应得到。当呋喃发生Mannich反应时,可以生成2-(N,N-二烷基氨基)甲基呋喃衍生物;在TMSI存在下,呋喃也可以与β-酮发生反应。而在Ag+催化下,1,3-二苯基硒丙烷(式2)或氯代环丙烷可与呋喃发生烯丙基化。
与自由基的反应 呋喃可以与亲电碳自由基反应生成2-烷基取代的呋喃(式3)。
有机金属化合物 在有机合成中,呋喃锂盐广泛使用。金属取代的呋喃能够与多种亲电试剂反应,并且2-锂代呋喃也可发生金属转移反应。2-取代呋喃的高价铜酸盐可以在低温下与酮进行共轭加成(式5)。
烯的环加成 在光照条件下,羰基化合物可以与呋喃进行[2+2]环加成生成环醚,经酸处理后可得到3-取代呋喃(式6)。此外,呋喃与氰氧化物可以进行[2+3]环加成(式7),用于合成多羟基胺类化合物。
二烯的环加成 当作为二烯参与Diels-Alder反应时,呋喃在有机合成中应用广泛。它不仅能与丙二烯化合物发生选择性产物反应,还能和乙炔类化合物反应,提供碳碳键形成的方法,并用于后续合成(式8)。此外,呋喃能通过与其他芳香烯的反应合成多烯化合物。
卡宾反应 包括卡宾加成生成2-氧-二环[3.1.0]己-3-烯等反应。当与乙烯基卡宾反应时,它会得到两种产物(式10)。
稳定性 稳定
禁配物 强氧化剂、酸类
避免接触条件 空气
聚合危害 不聚合
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:5
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:13.1
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:13 ppm (35.1 mg/m3)
-
危险品标志:T
-
安全说明:S45,S53,S61
-
危险类别码:R48/22,R52/53,R38,R20/22,R45,R12,R68,R19
-
WGK Germany:3
-
海关编码:2932999060
-
危险品运输编号:UN 2811 6.1/PG 2
-
危险类别:3
-
RTECS号:OB3870000
-
包装等级:I
-
储存条件:储存注意事项:通常商品含有阻聚剂。应将其存放在阴凉、通风的库房内,远离火种及热源。库温不宜超过29℃。避光保存,并确保包装密封,避免与空气接触。需与其他氧化剂和酸类分开存放,切忌混合储存。使用防爆型照明和通风设施,禁止使用可能产生火花的机械设备和工具。储区应配备泄漏应急处理设备及合适的收容材料。
SDS
| 国标编号: | 31040 |
| CAS: | 110-00-9 |
| 中文名称: | 呋喃 |
| 英文名称: | Furan;Divinylene oxide |
| 别 名: | 氧杂茂 |
| 分子式: | C 4 H 4 O;CHCHCHCHO |
| 分子量: | 68.07 |
| 熔 点: | -85.6℃ |
| 密 度: | 相对密度(水=1)0.94; |
| 蒸汽压: | -35℃ |
| 溶解性: | 不溶于水,溶于丙酮、苯,易溶于乙醇、乙醚等多数有机 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体,有温和的香味 |
| 危险标记: | 7(低闪点易燃液体) |
| 用 途: | 用于有机合成或用作溶剂 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:本品有麻醉和弱刺激作用。吸入后可引起头痛、头晕、血压下降、呼吸衰竭。
慢性影响:肝、肾损害。
二、毒理学资料及环境行为
急性毒性:LC 50 120mg/m 3 ,1小时(小鼠吸入)
危险特性:其蒸气与空气可形成爆炸性混合物。遇明火、高热能引起燃烧爆炸。与氧化剂能性强烈反应。在空气中能形成不稳定的过氧化物,蒸馏时易引起爆炸。本品与酸液接触,能发生强烈的放热反应。在火场中,受热的容器有爆炸危险。其蒸气比空气重,能在较低处扩散到相当远的地方,遇明火会引着回燃。
燃烧(分解)产物:一氧化碳、二氧化碳。
3.现场应急监测方法:
4.实验室监测方法:
比色法《化工企业空气中有害物质测定方法》,化学工业出版社
5.环境标准:
| 前苏联 | 车间空气中有害物质的最高容许浓度 | 0.5mg/m 3 |
| 前苏联(1975) | 水体中有害物质最高允许浓度 | 0.2mg/L |
| 前苏联 | 污水中有害物质最高允许浓度 | 20mg/L |
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源。防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
二、防护措施
呼吸系统防护:一般不需要特殊防护,高浓度接触时可佩戴自吸过滤式防毒面具(半面罩)。
眼睛防护:一般不需特殊防护。必要时,戴安全防护眼镜。
身体防护:穿防静电工作服。
手防护:戴防苯耐油手套。
其它:工作现场严禁吸烟。工作毕,淋浴更衣。注意个人清洁卫生。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
食入:饮足量温水,催吐,就医。
灭火方法:喷水冷却容器,可能的话将容器从火场移至空旷处。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:泡沫、干粉、二氧化碳、砂土。用水灭火无效。
制备方法与用途
呋喃亦称“氧(杂)茂”。分子式C4H4O,分子量68.07。无色液体,有特殊气味,放置变棕色,加少量水可延缓变色。固化点-86℃,沸点31.4℃,相对密度0.938,折光率1.4216。不溶于水,溶于乙醇、乙醚,易挥发,易燃烧,对碱稳定,对酸不稳定,与矿酸接触时聚合。其蒸气有麻醉性。暴露于空气中成过氧化物。
制法:由2-呋喃甲酸脱羧或直接将糠醛滴在熔融的氢氧化钠、氢氧化钾混合物上,也可通过蒸馏松香得到。用途:用于合成吡咯、四氢呋喃、噻吩等,用作溶剂,在药物中有多种呋喃衍生物如呋喃唑酮、呋喃妥因等。
化学性质呋喃是最简单的含氧五元杂环化合物,分子结构上含有一个环二烯醚的结构,属于6π电子系化合物,5个原子均为sp2杂化,处于同一平面。氧原子的一对未共用电子占据的p轨道垂直于环平面,与4个碳原子的p轨道形成π键,增强了稳定性。遇矿酸或加热蒸发能树脂化。
它易溶于乙醇、乙醚,溶于丙酮和苯,不溶于水。用途:主要用于制作大批量热芯盒工艺型芯的粘合剂。此外,呋喃可用来制取吡咯、噻吩、四氢呋喃等衍生物,四氢呋喃有许多重要用途。
生产方法:由糠醛氧化得到2-呋喃甲酸,经脱羧后即得呋喃。将2-呋喃甲酸加热至200-205℃(沸点左右),分解为呋喃和二氧化碳。反应过程中,升华的2-呋喃甲酸返回反应器,馏出的呋喃重新蒸馏,收集31-34℃馏分即得较纯的成品。收率约75%。
危害性挥发性高,沸点仅为31℃,空气中暴露极易通过生物膜被肺或肠吸收,引起头痛、头晕、恶心、呕吐,呼吸衰竭等症状,并对肝、肾等器官造成损伤,甚至诱发癌变。
物理性质无色液体。熔点85.65℃,沸点31.36℃,相对密度0.9514(20/4℃),折光率1.4214,闪点-35℃。易溶于乙醇、乙醚,溶于丙酮和苯,不溶于水。
用途主要用于制作大批量热芯盒工艺型芯的粘合剂。
生产方法由糠醛氧化得到2-呋喃甲酸,经脱羧后即得呋喃。反应过程中,升华的2-呋喃甲酸返回反应器,馏出的呋喃重新蒸馏,收集31-34℃馏分即得较纯的成品。收率约75%。
安全信息反应信息
-
作为反应物:参考文献:名称:吡咯及其制备方法摘要:一种吡咯及其制备方法,涉及化学制备领域。一种吡咯的制备方法,包括:在水蒸气存在的条件下,以固体超强酸为催化剂使呋喃与氨反应。该方法在固体超强酸作为催化剂的条件下,利用了价格较低且来源广泛的呋喃与氨反应制备吡咯,工艺流程简单,降低了反应温度,提高了产率,可批量生产。一种吡咯,由上述制备方法制备而成,产物纯度高。公开号:CN106831527A
-
作为产物:描述:cantharidin 在 palladium asbestos 作用下, 生成 呋喃参考文献:名称:v. Bruchhausen; Bersch, Archiv der Pharmazie, 1928, p. 701摘要:DOI:
-
作为试剂:参考文献:名称:Diaryliodonium盐在O芳构化中的竞争途径:机理见解。摘要:戴上戒指!已经使用实验技术和DFT计算研究了脂肪族醇和氢氧化物与二芳基碘鎓盐的芳基化,以得到烷基芳基醚和二芳基醚。观察到芳基形成和醇氧化途径与芳基化同时进行,并且发现了避免源自芳烃的副产物形成的添加剂。在与缺电子的二芳基碘鎓盐的反应中已经鉴定出新颖的直接亲核取代途径。DOI:10.1002/chem.201703057
文献信息
-
Combination of FBPase inhibitors and insulin sensitizers for the treatment of diabetes申请人:Metabasis Therapeutics, Inc.公开号:US06756360B1公开(公告)日:2004-06-29Pharmaceutical compositions containing an FBPase inhibitor and an insulin sensitizer are provided as well as methods for treating diabetes and diseases responding to increased glycemic control, an improvement in insulin sensitivity, a reduction in insulin levels, or an enhancement of insulin secretion.
-
Copper-Catalyzed Protodecarboxylation of Aromatic Carboxylic Acids作者:Lukas J. Gooßen、Werner R. Thiel、Nuria Rodríguez、Christophe Linder、Bettina MelzerDOI:10.1002/adsc.200700223日期:2007.10.8A catalyst generated from copper(I) oxide and 4,7-diphenyl-1,10-phenanthroline for the first time allows the catalytic protodecarboxylation even of deactivated aromatic carboxylic acids, giving rise to the corresponding arenes. Based on DFT calculations, a reaction pathway is proposed that accurately reflects the experimental results, such as the observed reactivity order of the substrates.
-
[EN] DERIVATIVES OF AMANITA TOXINS AND THEIR CONJUGATION TO A CELL BINDING MOLECULE<br/>[FR] DÉRIVÉS DE TOXINES D'AMANITES ET LEUR CONJUGAISON À UNE MOLÉCULE DE LIAISON CELLULAIRE申请人:HANGZHOU DAC BIOTECH CO LTD公开号:WO2017046658A1公开(公告)日:2017-03-23Derivatives of Amernita toxins of Formula (I), wherein, formula (a) R 1, R 2, R 3, R 4, R 5, R 6, R 7, R 8, R 9, R 10, X, L, m, n and Q are defined herein. The preparation of the derivatives. The therapeutic use of the derivatives in the targeted treatment of cancers, autoimmune disorders, and infectious diseases.
-
[EN] A CONJUGATE OF A TUBULYSIN ANALOG WITH BRANCHED LINKERS<br/>[FR] CONJUGUÉ D'UN ANALOGUE DE TUBULYSINE AVEC DES LIEURS RAMIFIÉS申请人:HANGZHOU DAC BIOTECH CO LTD公开号:WO2019127607A1公开(公告)日:2019-07-04The present invention relates to the conjugation of a tubulysin analog compound to a cell-binding molecule with branched/side-chain linkers for having better delivery of the conjugate compound and targeted treatment of abnormal cells. It also relates to a branched-linkage method of conjugation of a tubulysin analog molecule to a cell-binding ligand, as well as methods of using the conjugate in targeted treatment of cancer, infection and autoimmune disease.本发明涉及将一种管腔霉素类似物化合物与具有分支/侧链连接物的细胞结合分子结合,以实现结合物的更好传递和靶向治疗异常细胞。它还涉及一种将管腔霉素类似物分子与细胞结合配体结合的分支连接方法,以及在靶向治疗癌症、感染和自身免疫疾病中使用结合物的方法。
-
[EN] A CONJUGATE OF A CYTOTOXIC AGENT TO A CELL BINDING MOLECULE WITH BRANCHED LINKERS<br/>[FR] CONJUGUÉ D'UN AGENT CYTOTOXIQUE À UNE MOLÉCULE DE LIAISON CELLULAIRE AVEC DES LIEURS RAMIFIÉS申请人:HANGZHOU DAC BIOTECH CO LTD公开号:WO2020257998A1公开(公告)日:2020-12-30Provided is a conjugation of cytotoxic drug to a cell-binding molecule with a side-chain linker. It provides side-chain linkage methods of making a conjugate of a cytotoxic molecule to a cell-binding ligand, as well as methods of using the conjugate in targeted treatment of cancer, infection and immunological disorders.提供了一种将细胞毒性药物与一个侧链连接分子结合的共轭物。它提供了制备细胞毒性分子与细胞结合配体的共轭物的侧链连接方法,以及在靶向治疗癌症、感染和免疫性疾病中使用该共轭物的方法。
表征谱图
-
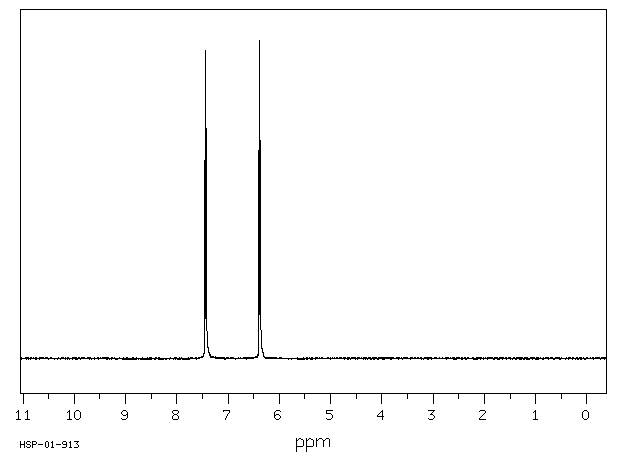
氢谱1HNMR
-
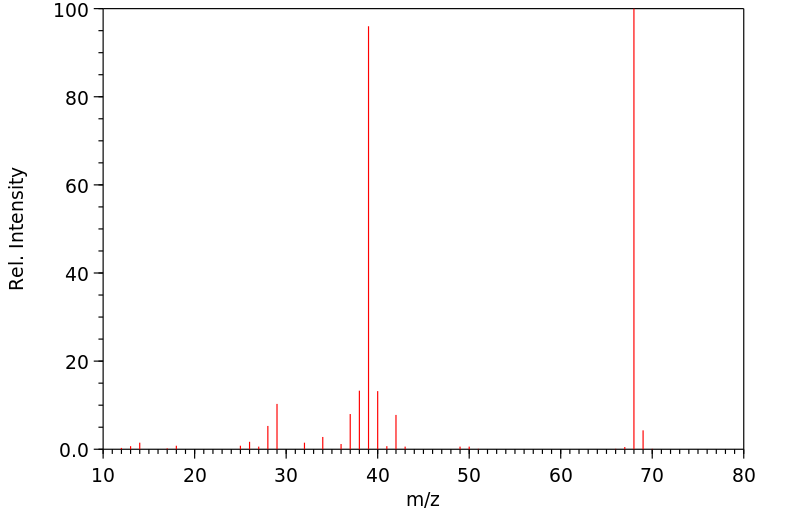
质谱MS
-
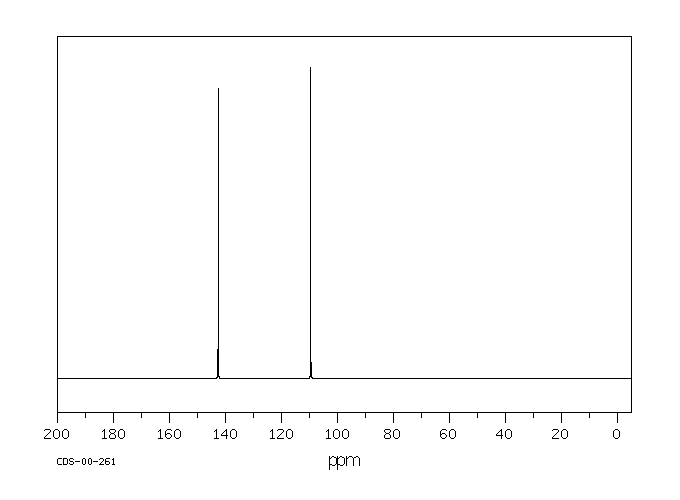
碳谱13CNMR
-
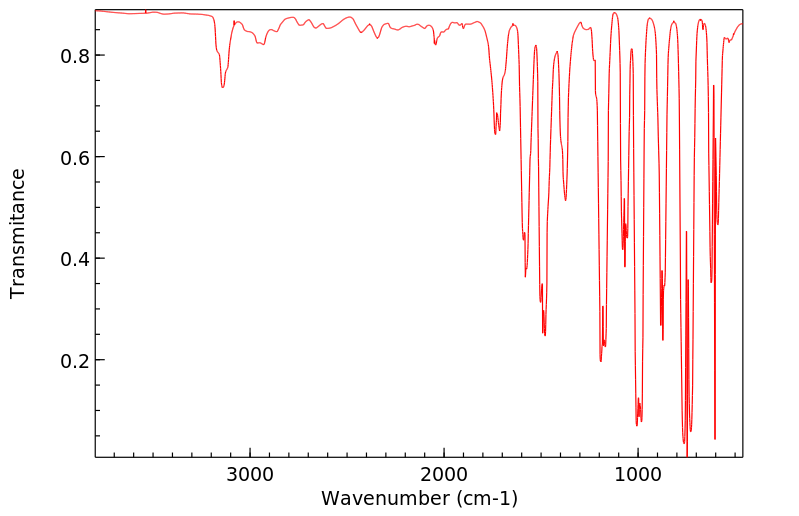
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息