1,9-二硝基吩嗪 | 58718-48-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:20
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:117
-
氢给体数:0
-
氢受体数:6
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 吩嗪-1,9-二胺 1,9-diaminophenazine 102877-14-5 C12H10N4 210.238
反应信息
-
作为反应物:参考文献:名称:Synthesis of novel acridino- and phenazino-18-crown-6 ligands and their optically pure dimethyl-substituted analogues for molecular recognition studies摘要:Novel acridino- and phenazino-18-crown-6 ligands 5 and 6 were I,prepared from acridine-4.5-diol (9) and phenazine-1,9-diol (10) with tetraethylene glycol di-p-tosylate (11) using potassium tert-butoxide as a bar in THF. New optically pure dimethyl-substituted acridino- and phenazine-18-crown-6 ligands (R,R)-7 and (R,R)-8 were also prepared by treating 9 and 10 with optically pure dimethyl-substituted tetraethylene glycol di-p-tosylate [(S,S)-18], Molecular recognition studies on these novel ligands are underway. (C) 1999 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4020(98)01128-4
-
作为产物:参考文献:名称:A Selective Biomimetic Tweezer for Noradrenaline摘要:The new highly preorganized tweezer molecule 1 binds noradrenaline in polar solvents with unprecedented specificity. It uses a biomimetic recognition pattern and rejects almost all other neurotransmitters. LB experiments on a film balance reflect the same selectivity if 1 is incorporated into a stearic acid monolayer.DOI:10.1021/ja035212l
文献信息
-
Decomposition pathways and mitigation strategies for highly-stable hydroxyphenazine flow battery anolytes作者:Nadeesha P. N. Wellala、Aaron Hollas、Kaining Duanmu、Vijayakumar Murugesan、Xin Zhang、Ruozhu Feng、Yuyan Shao、Wei WangDOI:10.1039/d1ta03655f日期:——undergoes desulfonation and reduction of a phenolic C–O bond to yield a mixture of 7/8-hydroxyphenazine-2-sulfonic acid, as well as hydrogenation of the aromatic ring system. Density functional theory (DFT) analysis of the charged DHPS, its ring-hydrogenated products, and variably substituted hydroxy phenazines has led to the development of a series of dihydroxylated phenazine isomers which provide insight水性有机氧化还原液流电池是一种很有前途的大规模储能技术。氧化还原活性有机分子的稳定性越来越被认为是主要障碍之一。在延长液流电池循环过程中,7,8-二羟基吩嗪-2-磺酸 (DHPS) 发生脱磺化和酚类 C-O 键的还原反应,生成 7/8-羟基吩嗪-2-磺酸的混合物,并氢化芳香环系统。带电 DHPS、其环氢化产物和可变取代的羟基吩嗪的密度泛函理论 (DFT) 分析导致了一系列二羟基吩嗪异构体的开发,这些异构体提供了对取代模式对溶解性和稳定性的影响的深入了解。合成了七种二羟基吩嗪 (DHP) 异构体及其溶解度,电化学性能、液流电池循环性能和降解途径已被研究。根据理论和实验结果,1、4、6 和 9 位的羟基取代产生高度稳定的衍生物,而 2、3、7 和 8 位的取代产生不稳定的衍生物。1,4- 和 1,6-DHP 与亚铁/铁氰化物结合的流通池实现了高稳定性,时间容量损失分别为每天 0.029% 和 0
-
Synthesis of phenazine derivatives for use as precursors to electrochemically generated bases作者:A. Mateo Alonso、Roberto Horcajada、Helen J. Groombridge、Reshma Chudasama (née Mandalia)、Majid Motevalli、James H. P. Utley、Peter B. WyattDOI:10.1039/b506295k日期:——1,6-Disubstituted phenazine derivatives for use as precursors to electrochemically generated bases have been synthesized from readily available starting materials. Reaction of 1,6-dihydroxyphenazine with 1,10-diododecane, 1,11-diiodo-3,6,9-trioxaundecane or (R,R)-(-)-1,2-bis(3-iodopropoxy)cyclohexane gave planar chiral phenazinophanes containing ether-linked bridges; molecular structures of all these
-
Pseudomonas fluorescens Showing Antifungal Activity against Macrophomina phaseolina, a Severe Pathogenic Fungus of Soybean, Produces Phenazine as the Main Active Metabolite作者:Stefany Castaldi、Marco Masi、Francisco Sautua、Alessio Cimmino、Rachele Isticato、Marcelo Carmona、Angela Tuzi、Antonio EvidenteDOI:10.3390/biom11111728日期:——
Pseudomonas fluorescens 9 and Bacillus subtilis 54, proposed as biofungicides to control Macrophomina phaseolina, a dangerous pathogen of soybean and other crops, were grown in vitro to evaluate their ability to produce metabolites with antifungal activity. The aim of the manuscript was to identify the natural compounds responsible for their antifungal activity. Only the culture filtrates of P. fluorescens 9 showed strong antifungal activity against M. phaseolina. Its organic extract contained phenazine and mesaconic acid (1 and 2), whose antifungal activity was tested against M. phaseolina, as well as Cercospora nicotianae and Colletotrichum truncatum, other pathogens of soybean; however, only compound 1 exhibited activity. The antifungal activity of compound 1 was compared to phenazine-1-carboxylic acid (PCA, 3), 2-hydroxyphenazine (2-OH P, 4), and various semisynthetic phenazine nitro derivatives in order to perform a structure–activity relationship (SAR) study. PCA and phenazine exhibited the same percentage of growth inhibition in M. phaseolina and C. truncatum, whereas PCA (3) showed lower activity against C. nicotianae than phenazine. 2-Hydroxyphenazine (4) showed no antifungal activity against M. phaseolina. The results of the SAR study showed that electron attractor (COOH and NO2) or repulsor (OH) groups significantly affect the antifungal growth, as well as their α- or β-location on the phenazine ring. Both PCA and phenazine could be proposed as biopesticides to control the soybean pathogens M. phaseolina, C. nicotianae, and C. truncatum, and these results should prompt an investigation of their large-scale production and their suitable formulation for greenhouse and field applications.
Pseudomonas fluorescens 9和Bacillus subtilis 54被提议作为生物杀菌剂,用于控制大豆和其他作物的危险病原体Macrophomina phaseolina。为了评估它们产生具有抗真菌活性的代谢物的能力,在体外培养了这些细菌。本文的目的是确定其抗真菌活性的自然化合物。只有P. fluorescens 9的培养滤液显示出对M. phaseolina的强烈抗真菌活性。其有机提取物含有苯肼和丙酮酸(1和2),对M. phaseolina、Cercospora nicotianae和Colletotrichum truncatum等大豆病原体的抗真菌活性进行了测试,但只有化合物1表现出活性。将化合物1的抗真菌活性与苯肼-1-羧酸(PCA,3)、2-羟基苯肼(2-OH P,4)以及各种半合成苯肼硝基衍生物进行比较,以进行结构-活性关系(SAR)研究。PCA和苯肼在M. phaseolina和C. truncatum中显示出相同的生长抑制百分比,而PCA(3)对C. nicotianae的活性低于苯肼。2-羟基苯肼(4)对M. phaseolina没有抗真菌活性。SAR研究的结果表明,电子吸引剂(COOH和NO2)或排斥剂(OH)基团显着影响抗真菌生长,以及它们在苯肼环上的α或β位置。PCA和苯肼都可以作为生物杀虫剂,用于控制大豆病原体M. phaseolina、C. nicotianae和C. truncatum,这些结果应促使对它们的大规模生产和适合温室和田间应用的适当配方进行研究。 -
Otomasu, Chemical and pharmaceutical bulletin, 1958, vol. 6, p. 77,80作者:OtomasuDOI:——日期:——
-
Maffei; Aymon, Gazzetta Chimica Italiana, 1954, vol. 84, p. 667,672作者:Maffei、AymonDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
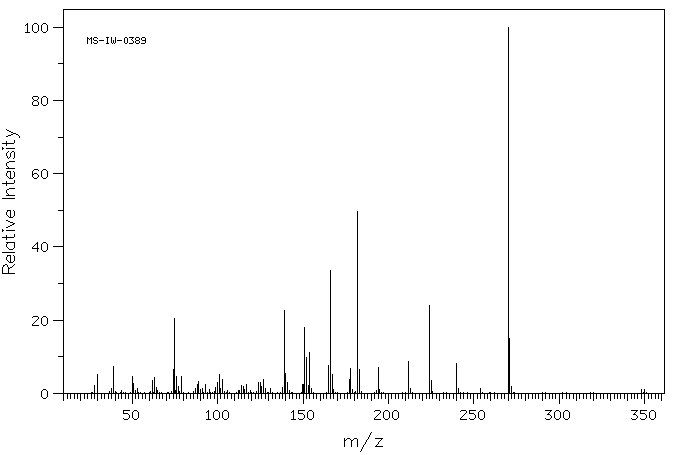
质谱MS
-
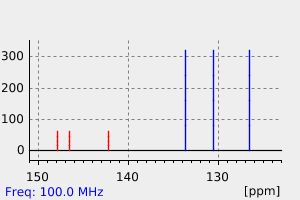
碳谱13CNMR
-
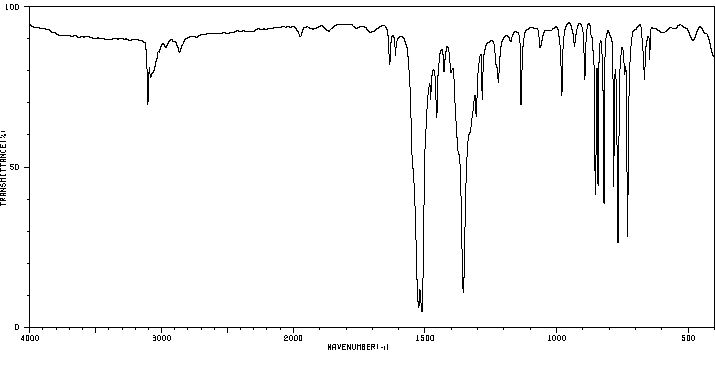
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息