2,3,4-三甲氧基苯甲腈 | 43020-38-8
中文名称
2,3,4-三甲氧基苯甲腈
中文别名
2,3,4-三甲氧基苄氰
英文名称
2,3,4-trimethoxybenzonitrile
英文别名
——
CAS
43020-38-8
化学式
C10H11NO3
mdl
MFCD00001785
分子量
193.202
InChiKey
YCSGHMDKBZNXJC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56-57 °C(lit.)
-
沸点:329.41°C (rough estimate)
-
密度:1.2307 (rough estimate)
-
溶解度:溶于甲醇
-
稳定性/保质期:
常规情况下不会分解,没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:51.5
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险品标志:Xn,T
-
安全说明:S26,S37/39
-
危险类别码:R23/24/25,R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:2926909090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:3276
-
储存条件:密封、阴凉、干燥保存
SDS
| Name: | 2 3 4-Trimethoxybenzonitrile 97% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 43020-38-8 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 43020-38-8 | 2,3,4-Trimethoxybenzonitrile | 97 | 256-049-5 |
Risk Phrases: 23/24/25
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation, in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. May cause cardiac disturbances. The toxicological properties of this substance have not been fully investigated. May cause central nervous system depression.
Metabolism may release cyanide, which may result in headache, dizziness, weakness, collapse, unconsciousness and possible death.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May cause cardiac abnormalities. May be metabolized to cyanide which in turns act by inhibiting cytochrome oxidase impairing cellular respiration.
Inhalation at high concentrations may cause CNS depression and asphixiation.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Chronic exposure to cyanide solutions may lead to the development of a "cyanide" rash, characterized by itching, and by macular, papular, and vesicular eruptions, and may be accompanied by secondary infections. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
May be partially metabolized to cyanide in the body.
Antidote: Always have a cyanide antidote kit on hand when working with cyanide compounds. Get medical advice to use.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Containers may explode in the heat of a fire. Non-combustible, substance itself does not burn but may decompose upon heating to produce irritating, corrosive and/or toxic fumes. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Substance is noncombustible; use agent most appropriate to extinguish surrounding fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 43020-38-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 56.00 - 57.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H11NO3
Molecular Weight: 193.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents, strong bases.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, cyanide fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 43020-38-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,3,4-Trimethoxybenzonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
Safety Phrases:
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 43020-38-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 43020-38-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 43020-38-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3,4-三甲氧基苯甲醛 2,3,4-trimethoxybenzaldehyde 2103-57-3 C10H12O4 196.203 2.3.4-三甲氧基苯乙酸 2,3,4-trimethoxyphenylacetic acid 22480-91-7 C11H14O5 226.229 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,3,4-三甲氧基苄胺 2,3,4-trimethoxybenzylamine 41219-16-3 C10H15NO3 197.234 2,3,4-三甲氧基苯甲酸 2,3,4-Trimethoxy-benzoic acid 573-11-5 C10H12O5 212.202
反应信息
-
作为反应物:描述:2,3,4-三甲氧基苯甲腈 在 [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 、 1-金刚烷甲酸 、 乙酰氯 作用下, 以 乙醇 为溶剂, 反应 20.0h, 生成 butyl 2-(3-ethoxy-4,5,6-trimethoxy-1H-isoindol-1-yl)acetate参考文献:名称:Ruthenium(II)-Catalyzed Redox-Neutral Oxidative Cyclization of Benzimidates with Alkenes with Hydrogen Evolution摘要:1H-Isoindoles and 2H-isoindoles are synthesized via a ruthenium-catalyzed oxidant-free cyclization of benzimidates with alkenes at room temperature with the liberation of H-2. Later, 1H-isoindoles were converted into nitrogen containing heterocycles. The proposed reaction mechanism was strongly supported by experimental evidence and DFT calculations.DOI:10.1021/acs.orglett.7b03405
-
作为产物:描述:2,3,4-三甲氧基苯甲醛 在 Mexican Bentonite 、 盐酸羟胺 作用下, 反应 0.25h, 以86%的产率得到2,3,4-三甲氧基苯甲腈参考文献:名称:在没有溶剂的情况下,使用墨西哥膨润土和微波或红外辐射从醛中直接合成芳香腈摘要:摘要 一种高效、简单的方法,用于通过红外或微波辐射,用盐酸羟胺和墨西哥膨润土将芳香醛直接转化为相应的腈。DOI:10.1080/00397919208021348
文献信息
-
Copper- and Silver-Mediated Cyanation of Aryl Iodides Using DDQ as Cyanide Source作者:Kui Zheng、Peng Yu、Shuyou Chen、Fen Chen、Jiang ChengDOI:10.1002/cjoc.201201140日期:2013.4A new copper and silver‐mediated cyanation of aryl iodides with DDQ as a cyanide source is achieved, providing nitriles with good yields. This new approach represents a safe method leading to aryl nitriles.
-
Iron and Phenol Co-Catalysis for Rapid Synthesis of Nitriles under Mild Conditions作者:Hong Meng、Sen Gao、Meiming Luo、Xiaoming ZengDOI:10.1002/ejoc.201900831日期:2019.7.31Using inexpensive and environmentally benign FeCl3 and 2,6‐di‐tert‐butyl‐4‐methylphenol as cooperative catalyst, benzoyl aldehyde oximes converted into nitriles in excellent yields within minutes under mild reaction conditions.使用廉价且对环境无害的FeCl 3和2,6-二叔丁基-4-甲基苯酚作为协同催化剂,在温和的反应条件下,苯甲酰醛肟在短短几分钟内即可以优异的收率转化为腈。
-
Stable and reusable nanoscale Fe<sub>2</sub>O<sub>3</sub>-catalyzed aerobic oxidation process for the selective synthesis of nitriles and primary amides作者:Kathiravan Murugesan、Thirusangumurugan Senthamarai、Manzar Sohail、Muhammad Sharif、Narayana V. Kalevaru、Rajenahally V. JagadeeshDOI:10.1039/c7gc02627g日期:——nitriles and amides from easily available starting materials using cost-effective catalysts and green reagents is highly desired. In this regard, herein we report the nanoscale iron oxide-catalyzed environmentally benign synthesis of nitriles and primary amides from aldehydes and aqueous ammonia in the presence of 1 bar O2 or air. Under mild reaction conditions, this iron-catalyzed aerobic oxidation process
-
Direct Carbon Isotope Exchange of Pharmaceuticals via Reversible Decyanation作者:Minghao Feng、Joao De Oliveira、Antoine Sallustrau、Gianluca Destro、Pierre Thuéry、Sebastien Roy、Thibault Cantat、Charles S. Elmore、Jorg Blankenstein、Frédéric Taran、Davide AudisioDOI:10.1021/jacs.1c01923日期:2021.4.21described. By utilizing the radiolabeled precursor Zn([14C]CN)2, this protocol allows the insertion of the desired carbon tag without the need for structural modifications, in a single step. By reducing synthetic costs and limiting the generation of radioactive waste, this procedure will facilitate the labeling of nitrile containing drugs and accelerate 14C-based ADME studies supporting drug development
-
Metal-Free One-Pot Conversion of Electron-Rich Aromatics into Aromatic Nitriles作者:Hideo Togo、Sousuke UshijimaDOI:10.1055/s-0029-1219575日期:2010.4Various electron-rich aromatics could be smoothly converted into the corresponding aromatic nitriles in good to moderate yields by treatment of electron-rich aromatics with POCl3 and DMF, followed by treatment with molecular iodine in aqueous ammonia. The present reaction is a novel metal-free one-pot method for the preparation of aromatic nitriles from electron-rich aromatics.
表征谱图
-
氢谱1HNMR
-
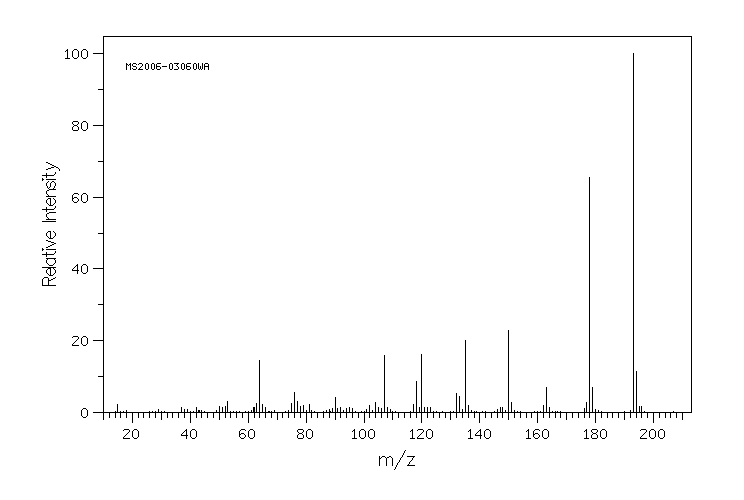
质谱MS
-
碳谱13CNMR
-
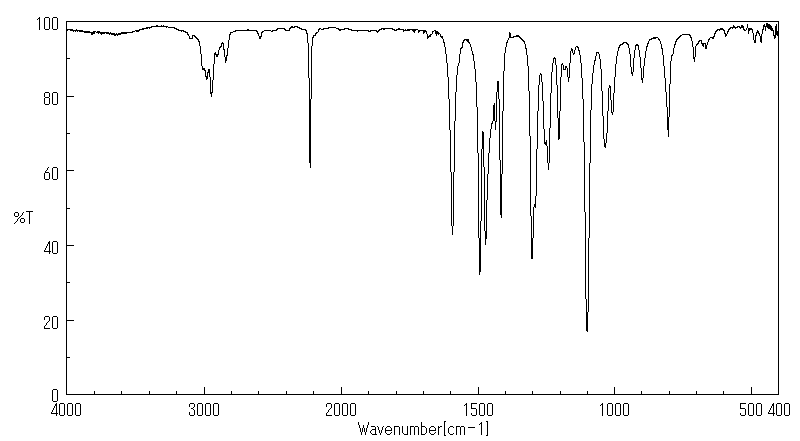
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫