2,3-二甲基苯基异硫氰酸酯 | 1539-20-4
物质功能分类
中文名称
2,3-二甲基苯基异硫氰酸酯
中文别名
2,3-二甲基异硫氰酸苯酯
英文名称
1-isothiocyanato-2,3-dimethylbenzene
英文别名
2,3-Xylylisothiocyanat;2,3-Dimethylphenylisothiocyanate
CAS
1539-20-4
化学式
C9H9NS
mdl
MFCD00041064
分子量
163.243
InChiKey
VASTZUGVKHOFPE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:22-23 °C
-
沸点:128 °C
-
密度:1.08
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知的危险反应,应避免接触氧化剂。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT, CORROSIVE
-
危险品标志:Xi,C
-
海关编码:2930909090
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境具有良好的通风或排气设施。
SDS
| Name: | 2 3-Dimethylphenyl isothiocyanate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1539-20-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1539-20-4 | 2,3-Dimethylphenyl isothiocyanate | 97% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye irritation. Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store under nitrogen.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1539-20-4: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H9NS
Molecular Weight: 163
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Strong oxidizing agents, alcohols, amines.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, oxides of sulfur, carbon dioxide, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1539-20-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,3-Dimethylphenyl isothiocyanate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 1539-20-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1539-20-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1539-20-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3-二甲基苯胺 2,3-Dimethylaniline 87-59-2 C8H11N 121.182
反应信息
-
作为反应物:描述:2,3-二甲基苯基异硫氰酸酯 在 sodium azide 、 盐酸 作用下, 以 乙醇 、 水 为溶剂, 反应 2.5h, 生成 1-(2,3-dimethyl-phenyl)-1H-tetrazole-5-thiol参考文献:名称:[EN] TETRAZOLE COMPOUNDS AS OREXIN RECEPTOR ANTAGONISTS
[FR] COMPOSÉS TÉTRAZOLIQUES COMME ANTAGONISTES DES RÉCEPTEURS À L'OREXINE摘要:该发明涉及式(I)的四唑化合物,其中X、Y、Z、R1、R2和R3如描述中所述;其药学上可接受的盐,以及将这些化合物用作药物,特别是促进睡眠药物的药物受体拮抗剂。公开号:WO2009150614A1 -
作为产物:描述:参考文献:名称:一些新的1,2,4-三唑和1,3,4-噻二唑衍生物的合成,抗氧化活性和脲酶抑制摘要:新系列的4,5-二取代-2,4-二氢-3 H -1,2,4-三唑-3-硫酮(8a – j)和2,5-二取代-1,3,4-噻二唑(9a – h)分别通过在4 N氢氧化钠水溶液中回流和与多磷酸搅拌过夜,通过肼基甲硫基酰胺衍生物(7a – k)的脱水环化反应合成。通过IR,1 H NMR,13 C NMR,元素分析和质谱研究表征了新合成的化合物的结构,并筛选了合成化合物的抗氧化和脲酶抑制活性。ñ-(2,4-二甲基苯基)-5-(4-硝基苯基)-1,3,4-噻二唑-2-胺(9h)显示出比标准药物更好的抗氧化活性,而4-(2,4-二甲基苯基) -5-(3-硝基苯基)-2,4-二氢-3 H -1,2,4-三唑-3-硫酮(8d)和4-(2,3-二甲基苯基)-5-苯基-2,4 -二氢-3 H -1,2,4-三唑-3-硫酮(8e)表现出强大的脲酶抑制活性。DOI:10.1016/j.ejmech.2010.08.034
文献信息
-
Synthesis and antitumor activity of novel pyridazinone derivatives containing 1,3,4-thiadiazole moiety作者:Junhu Qin、Mei Zhu、Hongmei Zhu、Liqiong Zhang、Yihong Fu、Jiamin Liu、Zhenchao Wang、Guiping OuYangDOI:10.1080/10426507.2020.1737062日期:2020.7.24-thiadiazol-2-yl)thio)pyridazin-3(2H)-One), it exhibited good anticancer activity on MGC-803 cells. Besides, introducing fluorine, chlorine, or trifluoromethyl group onto the benzene ring, such as compound 5 m (2-(Tert-butyl)−4-chloro-5-((5-((4-(trifluoromethoxy)phenyl)amino)−1,3,4-thiadiazol-2-yl)thio)pyridazin-3(2H)-One), displayed good anticancer activity on MGC-803 and Bcap-37 cells. Graphical Abstract摘要 合成了一系列含有1,3,4-噻二唑部分的新型哒嗪酮衍生物,并通过1H NMR、13C NMR、HRMS和IR光谱表征。其中,化合物5c的结构(2-(叔丁基)-4-氯-5-((5-((2-乙基苯基)氨基)-1,3,4-噻二唑-2-基)硫基)通过单晶 X 射线衍射分析明确证实了哒嗪-3(2H)-One)。通过MTT法测定所有目标化合物对MGC-803和Bcap-37的抑制活性,以阿霉素(抑制率分别为95.5±0.4%和95.7±1.0%)为对照。初步结果表明化合物5n(2-(叔丁基)-4-氯-5-((5-((3-氟苯基)氨基)-1,3,4-噻二唑-2-基) )thio)pyridazin-3(2H)-One) 优于其他。MGC-803和Bcap-37细胞在10 μmol/L浓度下的抑制率分别为86.3±2.2%和92.3±0.6%。初步的构效关系表明,当苯环的2-位被甲基取代时,如化合物
-
2-Imino-thiazolidin-4-one Derivatives as Potent, Orally Active S1P<sub>1</sub>Receptor Agonists作者:Martin H. Bolli、Stefan Abele、Christoph Binkert、Roberto Bravo、Stephan Buchmann、Daniel Bur、John Gatfield、Patrick Hess、Christopher Kohl、Céline Mangold、Boris Mathys、Katalin Menyhart、Claus Müller、Oliver Nayler、Michael Scherz、Gunther Schmidt、Virginie Sippel、Beat Steiner、Daniel Strasser、Alexander Treiber、Thomas WellerDOI:10.1021/jm100181s日期:2010.5.27through five specific G-protein coupled receptors numbered S1P1 through S1P5. Agonists of the S1P1 receptor block the egress of T-lymphocytes from thymus and lymphoid organs and hold promise for the oral treatment of autoimmune disorders. Here, we report on the discovery and detailed structure−activity relationships of a novel class of S1P1 receptor agonists based on the 2-imino-thiazolidin-4-one scaffold鞘氨醇-1-磷酸酯(S1P)是一种广泛的溶血磷脂,具有丰富的生物学效应。细胞外S1P通过五个特定的G蛋白偶联受体S1P 1至S1P 5传递其活性。S1P 1受体激动剂阻止T淋巴细胞从胸腺和淋巴器官流出,并有望用于自身免疫性疾病的口服治疗。在这里,我们报告的发现和详细的结构与活性之间的关系基于2-亚氨基-噻唑烷酮-4-酮骨架的新型S1P 1受体激动剂。化合物8bo(ACT- 128800)从该系列中出现,是一种有效的,选择性的,口服活性的S1P 1选择受体激动剂进行临床开发。在大鼠中,以3 mg / kg的剂量达到最大程度的循环淋巴细胞减少。淋巴细胞隔离的持续时间是剂量依赖性的。在100 mg / kg的剂量下,对淋巴细胞计数的影响在不到36小时内是完全可逆的。8bo在比格犬中的药代动力学研究表明,该化合物适合于人类每天一次给药。
-
Substituted 2-arylimino heterocycles and compositions containing them, for use as progesterone receptor binding agents申请人:Bayer Corporation公开号:US06353006B1公开(公告)日:2002-03-05This invention relates to 2-arylimino heterocycles, including 2-arylimino-1,3-thiazolidines, 2-arylimino-2,3,4,5-tetrahydro-1,3-thiazines, 2-arylimino-1,3-thiazolidin-4-ones, 2-arylimino-1,3-thiazolidin-5-ones, and 2-arylimino-1,3-oxazolidines, and their use in modulating progesterone receptor mediated processes, and pharmaceutical compositions for use in such therapies.
-
Facile and Versatile Synthesis of Alkyl and Aryl Isothiocyanates by Using Triphosgene and CoSolvent作者:Pengfei Liu、Chunyan Li、Jingwei Zhang、Xiaoyong XuDOI:10.1080/00397911.2013.783600日期:2013.12.17efficient method for the conversion of alkyl and aryl amines to isothiocyanates via dithiocarbamates has been developed using (CH3)2CO-CS2 as co-solvent and triphosgene as dehydrosulfurization reagent. High yields, mild reaction conditions and excellent functional group compatibility make it become a versatile synthetic method for the preparation of isothiocyanates compared with reported methods. [Supplementary
-
Synthesis of Novel Triazinoindole-Based Thiourea Hybrid: A Study on α-Glucosidase Inhibitors and Their Molecular Docking作者:Taha、Alshamrani、Rahim、Hayat、Ullah、Zaman、Imran、Khan、NazDOI:10.3390/molecules24213819日期:——
A new class of triazinoindole-bearing thiosemicarbazides (1–25) was synthesized and evaluated for α-glucosidase inhibitory potential. All synthesized analogs exhibited excellent inhibitory potential, with IC50 values ranging from 1.30 ± 0.01 to 35.80 ± 0.80 µM when compared to standard acarbose (an IC50 value of 38.60 ± 0.20 µM). Among the series, analogs 1 and 23 were found to be the most potent, with IC50 values of 1.30 ± 0.05 and 1.30 ± 0.01 µM, respectively. The structure–activity relationship (SAR) was mainly based upon bringing about different substituents on the phenyl rings. To confirm the binding interactions, a molecular docking study was performed.
表征谱图
-
氢谱1HNMR
-
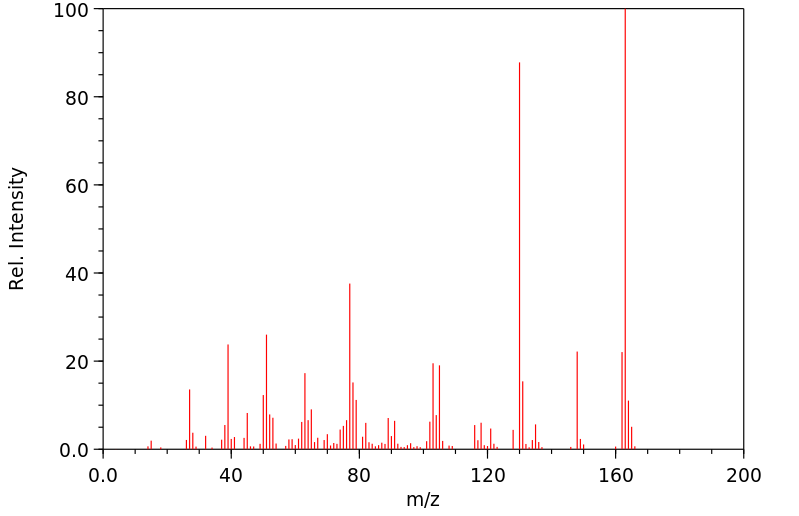
质谱MS
-
碳谱13CNMR
-
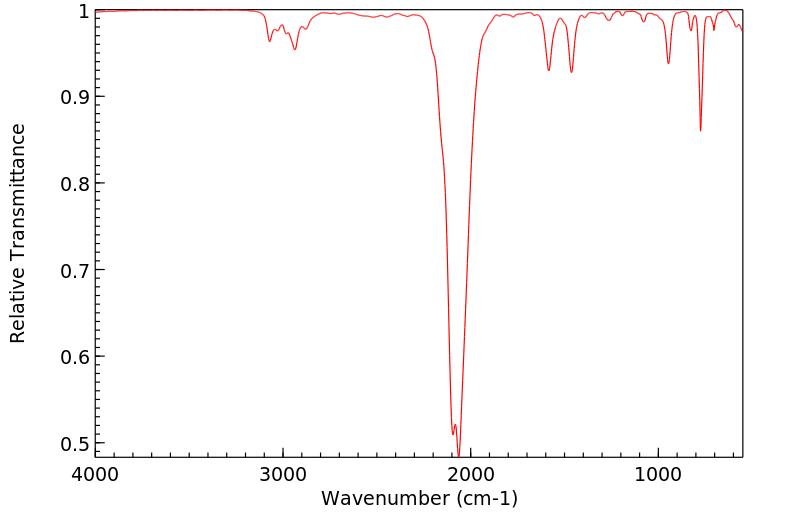
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫