2,4-二甲基己-2-烯 | 14255-23-3
中文名称
2,4-二甲基己-2-烯
中文别名
——
英文名称
2,4-dimethylhex-2-ene
英文别名
2,4-Dimethyl-hex-2-en;2,4-Dimethyl-2-hexene
CAS
14255-23-3
化学式
C8H16
mdl
——
分子量
112.215
InChiKey
IZSBIPQYBGDXJZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
反应信息
-
作为反应物:描述:4-苯基-1,2,4-三唑啉-3,5-二酮 、 2,4-二甲基己-2-烯 以 氯仿 为溶剂, 生成 1-(1-Isopropenyl-2-methyl-butyl)-4-phenyl-[1,2,4]triazolidine-3,5-dione参考文献:名称:三唑啉二酮与手性烯丙基醇的非对映选择性烯反应。亲羟基分子转向作用的证据摘要:PTAD与手性烯丙醇4-甲基-3-戊烯-2-醇的烯反应在非极性溶剂中表现出高的苏非对映选择性,而在极性溶剂中,非对映选择性显着降低。根据非对映异构体顺式和反叠氮基亚胺中间体的形成过程中羟基与传入的亲油体之间的导向作用来讨论这些结果。DOI:10.1016/s0040-4039(98)00207-x
-
作为产物:描述:2,4-二甲基-3-己酮 在 aluminum oxide 、 copper oxide-chromium oxide 作用下, 175.0~350.0 ℃ 、12.26 MPa 条件下, 生成 2,4-二甲基己-2-烯参考文献:名称:The Reaction of Mesityl Oxide with t-Butylmagnesium Chloride摘要:DOI:10.1021/ja01157a016
文献信息
-
Stereochemistry in the Ene Reactions of Singlet Oxygen and Triazolinediones with Allylic Alcohols. A Mechanistic Comparison作者:Georgios Vassilikogiannakis、Manolis Stratakis、Michael Orfanopoulos、Christopher S. FooteDOI:10.1021/jo990258b日期:1999.5.1The ene reaction of singlet oxygen with (Z)-4-methylpent-3-en-2-ol-2,5,5,5-d(4) (1-OH-d4) in nonpolar solvents exhibits a 90% three diastereoselectivity in the adduct derived from the major syn perepoxide intermediate, but also a moderate three diastereoselectivity in the adduct derived from the minor anti perepoxide. Photooxygenation of 2,4-dimethylpent-3-en-2-ol (2) exhibits a significant solvent dependence in the syn/anti methyl stereoselectivity, with nonpolar solvents promoting syn methyl reactivity, while polar solvents promote anti methyl reactivity. These results are in agreement with a steering effect between hydroxyl and singlet oxygen in the rate-determining step of the reaction. N-Phenyltriazolinedione addition to the chiral allylic alcohol 4-methylpent-3-en-2-ol (1-OH) is highly three diastereoselective in nonpolar solvents, with a solvent dependent variation in the threo/erythro ene products. On the other hand, the nonfunctionalized chiral alkene 2,4-dimethyl-2-hexene (1-Et) exhibits poor diastereoselectivity. Reaction of PTAD with 1-OH-d4 in nonpolar solvents, exhibits a significant three diastereoselectivity from the syn aziridinium imide intermediate, and a moderate three diastereoselectivity from the anti intermediate. These results are consonant with a steering effect between the hydroxyl and the electrophile, as proposed in the case of singlet oxygen addition to allylic alcohols 1-OH and 2. In contrast to the analogous O-1(2) ene reaction, a solvent independent ratio syn/anti similar to 50/50 was found in the addition of MTAD to 2. The intermolecular kinetic isotope effect in the reaction of 2 with MTAD (k(H)/k(D) = 1.15 +/- 0.02), is consistent with formation of the intermediate in fast step, indicative that the steering effect during the formation of aziridium imide is not important in the reaction kinetics. This energetic profile is in contrast to triazolinedione addition to the secondary allylic alcohol 1-OH, where the high three selectivity and the slight inverse kinetic isotope effect of k(H)/k(D) = 0.98 +/- 0.02 are consonant with the formation of the intermediate in the rate-determining step. An explanation for the increased reactivity of the syn methyl in the addition of MTAD to 2 (similar to 50%) is offered.
-
New triad alkylation reagent. Cross coupling of indium trialkyls with alkenyl halides作者:Ryoki Nomura、Shinichiro Miyazaki、Haruo MatsudaDOI:10.1021/ja00033a070日期:1992.3
表征谱图
-
氢谱1HNMR
-
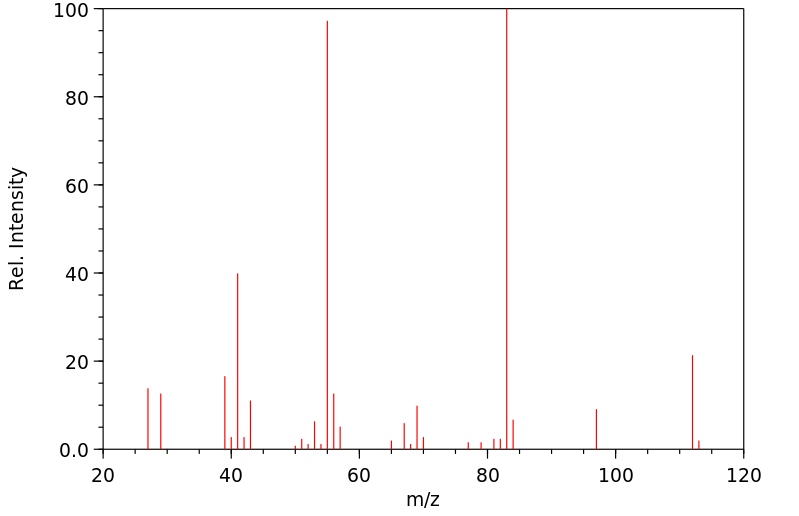
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-