2-烯丙基苯甲醚 | 4125-43-3
中文名称
2-烯丙基苯甲醚
中文别名
1-烯丙氧基-2-甲氧基苯;邻甲氧基苯基烯丙基醚
英文名称
O-allyl guaiacol
英文别名
1-(allyloxy)-2-methoxybenzene;allyl (2-methoxyphenyl) ether;Benzene, 1-methoxy-2-(2-propenyloxy)-;1-methoxy-2-prop-2-enoxybenzene
CAS
4125-43-3
化学式
C10H12O2
mdl
MFCD00026096
分子量
164.204
InChiKey
KWRBXILMRLLABD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:110-113 °C(Press: 12 Torr)
-
密度:1.0592 g/cm3
-
LogP:2.213 (est)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2909309090
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 2-ALLYLOXYANISOLE
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 4125-43-3
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 4), H302
Skin irritation (Category 2), H315
Serious eye damage (Category 1), H318
Specific target organ toxicity - single exposure (Category 3), H335
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xn Harmful R22, R37/38, R41
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Danger
Hazard statement(s)
H302 Harmful if swallowed.
H315 Causes skin irritation.
H318 Causes serious eye damage.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P280 Wear protective gloves/ eye protection/ face protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Formula : C10H12O2
Molecular Weight : 164,21 g/mol
CAS-No. : 4125-43-3
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
2-ALLYLOXYANISOLE
Acute Tox. 4; Skin Irrit. 2; Eye -
Dam. 1; STOT SE 3; H302,
H315, H318, H335
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapours, mist or gas. Ensure adequate ventilation.
Evacuate personnel to safe areas.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Soak up with inert absorbent material and dispose of as hazardous waste. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Normal measures for preventive fire protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Tightly fitting safety goggles. Faceshield (8-inch minimum). Use equipment for eye protection
tested and approved under appropriate government standards such as NIOSH (US) or EN
166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 2,638
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Specific target organ toxicity - single exposure
May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Full text of H-Statements referred to under sections 2 and 3.
Acute Tox. Acute toxicity
Eye Dam. Serious eye damage
H302 Harmful if swallowed.
H315 Causes skin irritation.
H318 Causes serious eye damage.
H335 May cause respiratory irritation.
Skin Irrit. Skin irritation
STOT SE Specific target organ toxicity - single exposure
Full text of R-phrases referred to under sections 2 and 3
R22 Harmful if swallowed.
R37/38 Irritating to respiratory system and skin.
R41 Risk of serious damage to eyes.
Further information
Copyright 2013 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 木榴油 2-methoxy-phenol 90-05-1 C7H8O2 124.139 —— 1-methoxy-2-[(Z)-prop-1-enoxy]benzene 51896-39-0 C10H12O2 164.204 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methoxy-2-n-propoxybenzene 29515-39-7 C10H14O2 166.22 2-(2-甲氧基苯氧基)乙醛 (2-methoxyphenoxy)acetaldehyde 18167-91-4 C9H10O3 166.177 —— (R)-guaifenesin 61248-75-7 C10H14O4 198.219 —— (S)-guaifenesin 61248-76-8 C10H14O4 198.219 —— (S)-1-Amino-3-(2-methoxy-phenoxy)-propan-2-ol 61248-82-6 C10H15NO3 197.234 木榴油 2-methoxy-phenol 90-05-1 C7H8O2 124.139 愈创木酚缩水甘油醚 2-(2-methoxy-phenoxymethyl)-oxirane 2210-74-4 C10H12O3 180.203 (S)-2-甲氧基苯基缩水甘油醚 (S)-2-(2,3-epoxypropoxy)anisole 61248-99-5 C10H12O3 180.203 —— ethyl 4-(2-methoxyphenoxy)butanoate 56359-21-8 C13H18O4 238.284 —— 1-methoxy-2-[(Z)-prop-1-enoxy]benzene 51896-39-0 C10H12O2 164.204 左莫普洛尔 (S)-(-)-moprolol 77164-20-6 C13H21NO3 239.315 - 1
- 2
反应信息
-
作为反应物:描述:2-烯丙基苯甲醚 在 palladium diacetate 、 sodium hydride 作用下, 以 N,N-二甲基乙酰胺 为溶剂, 反应 4.0h, 以91%的产率得到木榴油参考文献:名称:钯催化氢化钠对醚和酯的脱苄基作用和去甲酰化作用摘要:在这里,我们简单地证明,添加Pd(OAc)2作为促进剂会将常用的碱式NaH的反应性切换为亲核还原剂。反应性被设计成钯催化的芳基醚和酯的还原性脱苄基作用和脱甲酰化作用。该操作简单,温和的方案显示了广泛的底物范围和宽泛的官能团耐受性(> 50个实例),并且对脂族结构上的芳基醚具有高化学选择性。此外,在有效的合成工艺中证明了NaH作为碱和还原剂的双重反应性。DOI:10.1021/acscatal.8b00185
-
作为产物:描述:参考文献:名称:Thermodynamic, spectroscopic, and density functional theory studies of allyl aryl and prop-1-enyl aryl ethers. Part 1. Thermodynamic data of isomerization摘要:通过对70对异构化的烯丙基苯基醚(a)和(Z)-丙-1-烯基苯基醚(b)在DMSO溶液中的化学平衡研究,考察了它们相对热力学稳定性的变化。从平衡常数随温度的变化中,评估了在298.15 K下的异构化吉布斯自由能、焓和熵。由于其低焓值,(Z)-丙-1-烯基苯基醚在平衡状态下具有显著优势,a→b异构化的吉布斯自由能范围从-12至-23 kJ/mol。在大多数反应中,熵的贡献可以忽略不计,但偶尔会发现小于+10 J/K·mol的正值。平衡研究还扩展到涉及两对在烯键C(2)位置带有甲基取代基的相关异构化醚。甲基取代基可以增加烯丙基醚的相对热力学稳定性约3.4 kJ/mol。DOI:10.1039/b101837j
文献信息
-
1,2-benzisoxazole compounds申请人:Adir et Compagnie公开号:US05100902A1公开(公告)日:1992-03-31Compounds of formula I ##STR1## in which m represents an integer from 0 to 5, n represents an integer from 1 to 2, p is equal to 0, 1 or 2, X, Y and Z, which may be identical or different, each represent a hydrogen atom, a halogen atom, a linear or branched alkyl radical, a trifluoromethyl radical, an alkoxy radical, an alkylthio radical or a hydroxyl radical, and R represents a 2-benzofuranyl or 2,3-dihydro-2-benzofuranyl radical (it being possible for each to be substituted on the benzene ring), a 2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran-2-yl radical, a 4-oxo-4H-chromen-2-yl radical (optionally substituted on the benzene ring), a benzocyclobutenyl radical of formula A or an indanyl radical of formula B: ##STR2## (in which: R.sub.1 and R.sub.2, which may be identical or different, each represent a hydrogen atom, a halogen atom, a trifluoromethyl radical, an alkyl radical, an alkoxy radical, a hydroxyl radical, a hydroxyalkyl radical or an alkylthio radical, or together form a methylenedioxy radical or an ethylenedioxy radical, and R.sub.3 represents a hydrogen atom or a linear or branched alkyl radical having 1 to 6 carbon atoms), or a radical of formula C: ##STR3## (in which R.sub.4, R.sub.5 and R.sub.6, which may be identical or different, each represent a hydrogen atom, a halogen atom, a trifluoromethyl radical, a hydroxyl radical, an alkyl radical, an alkoxy radical or an alkylthio radical, their optical isomers and their addition salts with a pharmaceutically acceptable organic or inorganic acid.式I的化合物##STR1##中,其中m表示0到5的整数,n表示1到2的整数,p等于0、1或2,X、Y和Z,可以相同也可以不同,每个代表氢原子、卤素原子、直链或支链烷基基团、三氟甲基基团、烷氧基团、烷基硫基团或羟基基团,而R代表2-苯并呋喃基或2,3-二氢-2-苯并呋喃基(每个都可以在苯环上被取代),2,3,6,7-四氢苯并[1,2-b:4,5-b']二呋喃-2-基基团,4-氧代-4H-香豆素-2-基基团(在苯环上可选择取代),A式苯并环丁烯基基团或B式茚基基团:##STR2##(其中:R.sub.1和R.sub.2,可以相同也可以不同,每个代表氢原子、卤素原子、三氟甲基基团、烷基基团、烷氧基团、羟基基团、羟基烷基基团或烷基硫基团,或者一起形成亚甲二氧基基团或乙烯二氧基基团,而R.sub.3代表氢原子或具有1到6个碳原子的直链或支链烷基基团),或C式基团:##STR3##(其中R.sub.4、R.sub.5和R.sub.6,可以相同也可以不同,每个代表氢原子、卤素原子、三氟甲基基团、羟基基团、烷基基团、烷氧基团或烷基硫基团,它们的光学异构体及其与药学上可接受的有机或无机酸的加合盐。
-
A Diastereoselective Route to<i>trans</i>-2-Aryl-2,3-dihydrobenzofurans through Sequential Cross-Metathesis/Isomerization/Allylboration Reactions: Synthesis of Bioactive Neolignans作者:Rémy Hemelaere、François Carreaux、Bertrand CarboniDOI:10.1002/ejoc.201500019日期:2015.4anti-homoallylic alcohols allow the synthesis of the desired skeleton in a stereoselective fashion. As an illustration, we used this strategy for the preparation of the dihydrodehydrodiconiferyl alcohol (1a), a natural dihydrobenzofuran neolignan, as well as for a formal synthesis of its O-demethylated derivative 1b. An enantioselective version of this approach employing a chiral phosphoric acid in the
-
Subsupercritical Water Generated by Inductive Heating Inside Flow Reactors Facilitates the Claisen Rearrangement作者:Andreas Kirschning、Mona OltmannsDOI:10.1055/s-0040-1705945日期:2020.12O-allylated phenols, including fluorine-modified phenols, is facilitated in aqueous media at high temperatures and pressures under flow conditions, as opposed to organic solvents. The O-allylation of phenols can be coupled with the Claisen rearrangement in an integrated flow system. Publication History Received: 14 August 2020 Accepted after revision: 16 September 2020 Publication Date: 27 October 2020
-
An efficient intermolecular BINAM–copper(I) catalyzed Ullmann-type coupling of aryl iodides/bromides with aliphatic alcohols作者:Ajay B. Naidu、G. SekarDOI:10.1016/j.tetlet.2008.03.015日期:2008.5wide range of alkyl aryl ethers are synthesized from the corresponding aryl iodides and aliphatic alcohols through Ullmann-type intermolecular coupling reactions in the presence of a catalytic amount of easily available BINAM–CuI complex. Less reactive aryl bromides have also been shown to react with aliphatic alcohols under identical reaction conditions to give good yields of the alkyl aryl ethers without
-
Enantioselective synthesis of benzofurans and benzoxazines via an olefin cross-metathesis–intramolecular oxo-Michael reaction作者:Jun-Wei Zhang、Quan Cai、Qing Gu、Xiao-Xin Shi、Shu-Li YouDOI:10.1039/c3cc43937b日期:——Chiral phosphoric acid and HoveydaâGrubbs II were found to catalyze an olefin cross-metathesisâintramolecular oxo-Michael cascade reaction of the ortho-allylphenols and enones to provide a variety of benzofuran and benzoxazine derivatives in moderate to good yields and enantioselectivity.
表征谱图
-
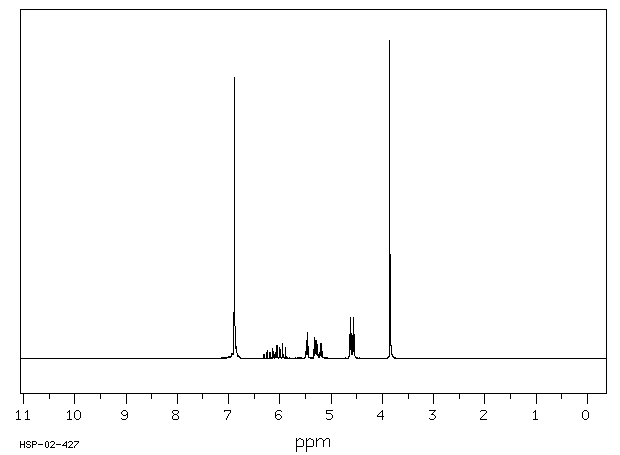
氢谱1HNMR
-
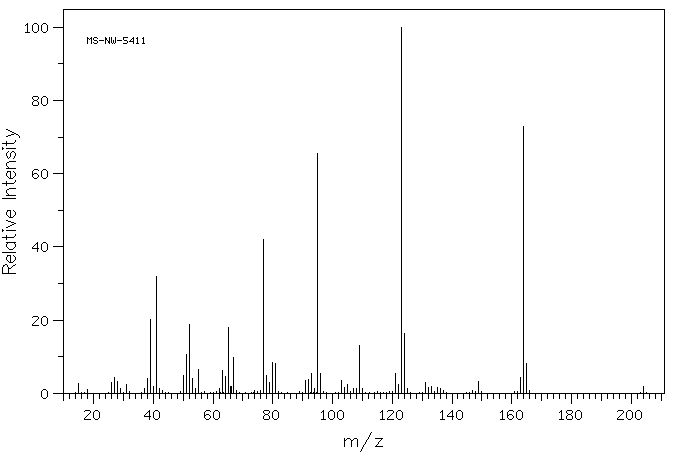
质谱MS
-
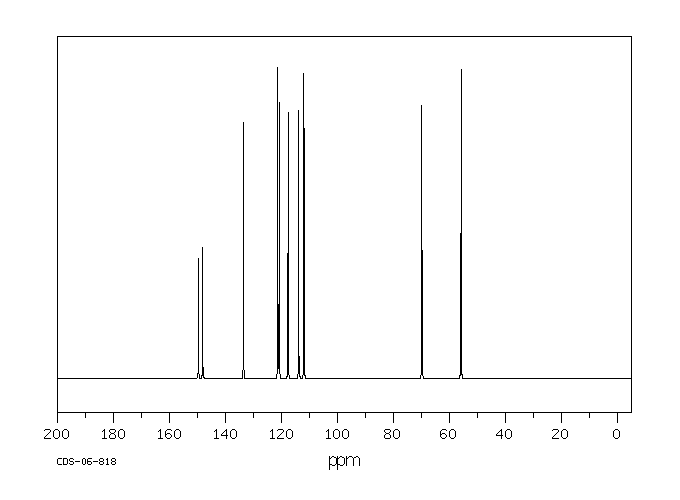
碳谱13CNMR
-
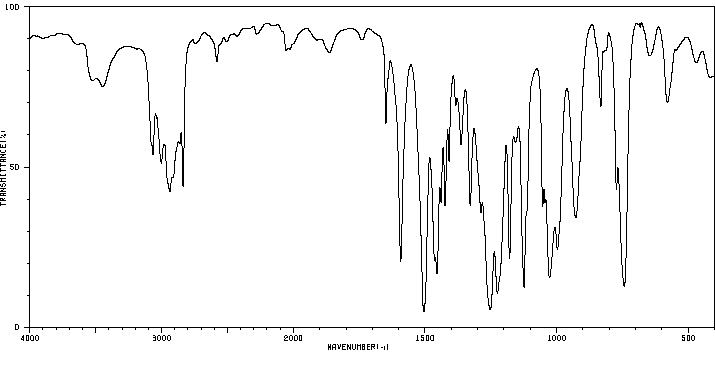
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯