3,5-二甲基环己醇 | 5441-52-1
中文名称
3,5-二甲基环己醇
中文别名
3,5-二甲基己醇;3,5-二甲基环己醇(异构体的混合物)
英文名称
3,5-dimethylcyclohexan-1-ol
英文别名
3,5-dimethyl-cyclohexanol;3,5-Dimethylcyclohexanol
CAS
5441-52-1
化学式
C8H16O
mdl
——
分子量
128.214
InChiKey
WIYNOPYNRFPWNB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:11-12 °C(lit.)
-
沸点:185-186 °C(lit.)
-
密度:0.892 g/mL at 25 °C(lit.)
-
闪点:164 °F
-
溶解度:在甲醇中几乎透明
-
LogP:2.353 (est)
-
保留指数:972;974
-
稳定性/保质期:
常温常压下,该物质为稳定液体,通常以立体异构体的混合物存在。其相对密度约为0.892。熔点在11~12℃之间,沸点则在185~186℃范围内。折光率为1.4550。闪点为73℃。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S23,S24/25
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温、避光、存放在阴凉干燥处,并密封保存。
SDS
1.1 产品标识符
: 3,5-二甲基环己醇
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
易燃液体 (类别4)
2.2 GHS 标记要素,包括预防性的陈述
象形图 无
警示词 警告
危险申明
H227 可燃液体
警告申明
预防
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
措施
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
储存
P403 + P235 存放在通风良好的地方。保持低温。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C8H16O
分子式
: 128.21 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
禁止催吐。 切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
小(起始)火时,使用媒介物如“乙醇”泡沫、干化学品或二氧化碳。大火时,尽可能使用水灭火。使用大量(
洪水般的)水以喷雾状应用;水柱可能是无效的。用大量水降温所有受影响的容器。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
水喷雾可用来冷却未打开的容器。
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。 移去所有火源。 防范蒸汽积累达到可爆炸的浓度,蒸汽能在低洼处积聚。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第13部分)。
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
防止吸入蒸汽和烟雾。
切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 11 - 12 °C - lit.
f) 起始沸点和沸程
185 - 186 °C - lit.
g) 闪点
74 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
0.892 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
热,火焰和火花。
10.5 不兼容的材料
强氧化剂, 酰氯, 酸酐
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
此易爆炸产品可以在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 3,5-二甲基环己醇
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
易燃液体 (类别4)
2.2 GHS 标记要素,包括预防性的陈述
象形图 无
警示词 警告
危险申明
H227 可燃液体
警告申明
预防
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
措施
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
储存
P403 + P235 存放在通风良好的地方。保持低温。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C8H16O
分子式
: 128.21 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
禁止催吐。 切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
小(起始)火时,使用媒介物如“乙醇”泡沫、干化学品或二氧化碳。大火时,尽可能使用水灭火。使用大量(
洪水般的)水以喷雾状应用;水柱可能是无效的。用大量水降温所有受影响的容器。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
水喷雾可用来冷却未打开的容器。
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。 移去所有火源。 防范蒸汽积累达到可爆炸的浓度,蒸汽能在低洼处积聚。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第13部分)。
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
防止吸入蒸汽和烟雾。
切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 11 - 12 °C - lit.
f) 起始沸点和沸程
185 - 186 °C - lit.
g) 闪点
74 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
0.892 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
热,火焰和火花。
10.5 不兼容的材料
强氧化剂, 酰氯, 酸酐
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
此易爆炸产品可以在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
用途
用于有机合成。
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:铁催化剂上醇和酮的有效气相脱氧摘要:讨论了一种在600 K和1-2·10 5 Pa下铁催化剂上将醇和酮气相脱氧为烃的方法。DOI:10.1016/s0040-4039(00)98301-1
-
作为产物:描述:3,5-二甲基-2-环己烯-1-酮 在 palladium 10% on activated carbon 、 氢气 、 borane tert-butylamine 作用下, 以 甲醇 、 二氯甲烷 为溶剂, 25.0~35.0 ℃ 、2.5 MPa 条件下, 反应 1.0h, 生成 3,5-二甲基环己醇参考文献:名称:一种顺,顺-3,5-二甲基-1-环己醇的制备方法摘要:本发明属于化学领域,公开了一种如式(I)所示的顺,顺‑3,5‑二甲基‑1‑环己醇化合物的合成方法,以乙醛和乙酰乙酸乙酯为原料,经knoevenagel缩合、水解、脱羧、加氢还原、还原、酰氯、水解等一系列反应合成得到顺,顺‑3,5‑二甲基‑1‑环己醇。该路线原辅料简单易得,反应条件温和,操作简便,合成成本低廉,得到的产品手性纯度高(产品/异构体比例=30:1~100:1),适于大规模生产。公开号:CN111484393A
-
作为试剂:描述:6-methyl-hexadecanoic acid ethyl ester 在 3,5-二甲基环己醇 、 sodium 、 xylene 作用下, 生成 6-methyl-hexadecan-1-ol参考文献:名称:Weitzel; Wojahn, Hoppe-Seyler's Zeitschrift fur Physiologische Chemie, 1951, vol. 287, p. 296,304,305摘要:DOI:
文献信息
-
Oxidation of alcohols using dimethyl sulfoxide and trichloromethyl chloroformate作者:Seiichi Takano、Kohei Inomata、Shun'ichi Tomita、Masashi Yanase、Kiyohiro Samizu、Kunio OgasawaraDOI:10.1016/s0040-4039(00)82412-0日期:1988.1Treatment of structurally diverse alcohols with dimethyl sulfoxide in the presence of trichloromethyl chloroformate (phosgene dimer) and triethylamine efficiently afforded the corresponding aldehydes or ketones in excellent yields.
-
Selective Catalytic Hydrogenation of Arenols by a Well-Defined Complex of Ruthenium and Phosphorus–Nitrogen PN<sup>3</sup>–Pincer Ligand Containing a Phenanthroline Backbone作者:Huaifeng Li、Yuan Wang、Zhiping Lai、Kuo-Wei HuangDOI:10.1021/acscatal.7b01316日期:2017.7.7Selective catalytic hydrogenation of aromatic compounds is extremely challenging using transition-metal catalysts. Hydrogenation of arenols to substituted tetrahydronaphthols or cyclohexanols has been reported only with heterogeneous catalysts. Herein, we demonstrate the selective hydrogenation of arenols to the corresponding tetrahydronaphthols or cyclohexanols catalyzed by a phenanthroline-based
-
A Ni–Mg–Al layered triple hydroxide-supported Pd catalyst for heterogeneous acceptorless dehydrogenative aromatization作者:Xiongjie Jin、Kento Taniguchi、Kazuya Yamaguchi、Kyoko Nozaki、Noritaka MizunoDOI:10.1039/c7cc01182b日期:——
In the presence of a Ni–Mg–Al layered triple hydroxide-supported Pd catalyst, the acceptorless dehydrogenative aromatization of a wide range of substrates efficiently proceeded with the liberation of molecular hydrogen.
-
Copper nanoparticles on hydrotalcite as a heterogeneous catalyst for oxidant-free dehydrogenation of alcohols作者:Takato Mitsudome、Yusuke Mikami、Kaori Ebata、Tomoo Mizugaki、Koichiro Jitsukawa、Kiyotomi KanedaDOI:10.1039/b809012b日期:——We have developed a highly efficient heterogeneous catalytic system using hydrotalcite-supported Cu nanoparticles (Cu/HT) that can successfully promote the oxidant-free dehydrogenation of various alcohols under liquid-phase conditions.
-
Phosphine Effects in the Copper(I) Hydride-Catalyzed Hydrogenation of Ketones and Regioselective 1,2-Reduction of α,β-Unsaturated Ketones and Aldehydes. Hydrogenation of Decalin and Steroidal Ketones and Enones作者:Jian-Xin Chen、John F. Daeuble、Jeffrey M. StrykerDOI:10.1016/s0040-4020(00)00133-2日期:2000.4The stereoselectivity and regioselectivity of the catalytic hydrogenation of ketones and α,β-unsaturated ketones and aldehydes using soluble copper(I) hydride catalysts have been investigated as a function of the ancillary phosphine ligand. While a relatively narrow range of aryldialkylphosphine ligands produce active hydrogenation catalysts, some ligands provide higher selectivity for 1,2-reduction
表征谱图
-
氢谱1HNMR
-
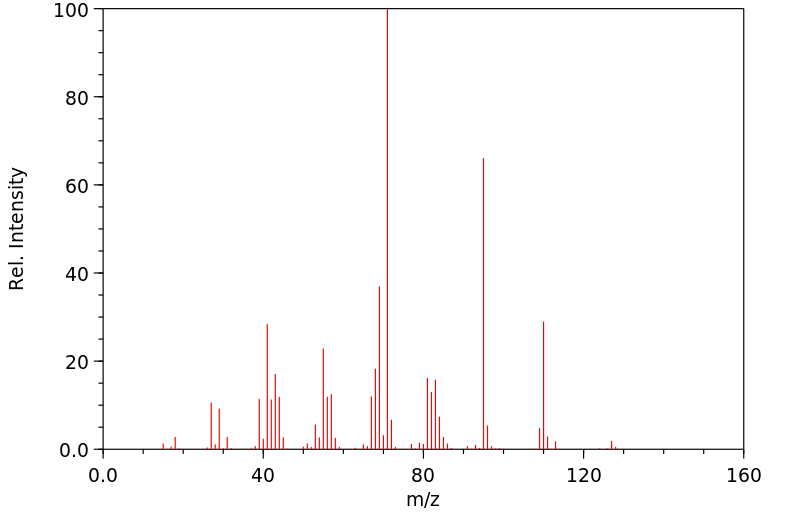
质谱MS
-
碳谱13CNMR
-
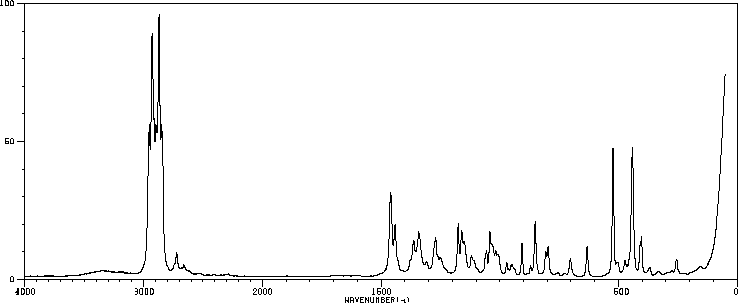
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷