甲苯 | 108-88-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
-
在强氧化剂如高锰酸钾、重铬酸钾、硝酸的作用下,甲苯会被氧化成苯甲酸。在催化剂存在下,使用空气或氧气也可以实现这一转化。在硫酸的存在下,于40℃以下用二氧化锰进行氧化反应会生成苯甲醛。当镍或铂作为催化剂时,在还原条件下可得到甲基环己烷。采用三氯化铝或三氯化铁作催化剂,甲苯与卤素反应会生成邻位和对位的卤代甲苯。加热并光照下,甲苯与卤素发生反应,则会产生苄基卤;与硝酸作用则生成邻位和对位的硝基甲苯。若用混酸(硫酸+硝酸)进行硝化反应可得到2,4-二硝基甲苯;进一步硝化会生成2,4,6-三硝基甲苯,即TNT。此外,在浓硫酸或发烟硫酸的作用下,甲苯会发生磺化反应形成邻位和对位的甲基苯磺酸。在三氯化铝或三氟化硼催化作用下,甲苯可以与卤代烃、烯烃、醇进行烷基化反应生成多种烷基甲苯混合物。而通过甲醛和盐酸的作用,甲苯会发生氯甲基化反应生成邻位或对位的甲基苄基氯。
-
稳定性:甲苯性质稳定。
-
禁配物:强氧化剂、酸类、卤素等。
-
聚合危害:不会发生聚合。
-
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
制备方法与用途
-
煤焦化副产的粗苯馏分中含甲苯15-20%,曾经是甲苯主要来源,生产每吨焦炭可回收甲苯1.1-1.3kg。从20世纪50年代开始,世界甲苯的主要来源已由煤焦化副产转变为催化重整和烃类裂解;到1982年,石油甲苯的产量已占总产量的96%以上。催化重整油中含芳烃50-60%,其中甲苯含量可达40-45%;裂解汽油中的芳烃含量为70%(质量)左右,其中15-20%是甲苯。
-
另一种方法是在甲苯中慢慢加入98%的硫酸,混匀并使噻吩反应完全后静置0.5小时,分出下层酸液。当硫酸试验合格时即表示酸洗结束;然后用蒸馏水充分洗涤至硫化合物含量合格,并用水洗至酸碱度合格,最后通过无水氯化钙脱水,进行最终的精馏处理以获得纯甲苯。
甲苯是芳香族碳氢化合物的一员,其性质与苯相似。在工业中常被用作有机溶剂及有毒性苯的替代品,并作为许多化工产品的原料,包括但不限于制造炸药、农药、苯甲酸、染料和合成树脂等。此外,它还是一种重要的汽油组分。
用途上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对二甲苯 para-xylene 106-42-3 C8H10 106.167 间二甲苯 m-xylene 108-38-3 C8H10 106.167 均三甲苯 1,3,5-trimethyl-benzene 108-67-8 C9H12 120.194 邻二甲苯 o-xylene 95-47-6 C8H10 106.167 —— benzylidenamine 16118-22-2 C7H7N 105.139 苄胺 benzylamine 100-46-9 C7H9N 107.155 苯甲醛 benzaldehyde 100-52-7 C7H6O 106.124 苯甲醇 benzyl alcohol 100-51-6 C7H8O 108.14 苄基碘 iodomethylbenzene 620-05-3 C7H7I 218.037 苄硫醇 phenylmethanethiol 100-53-8 C7H8S 124.207 苯甲腈 benzonitrile 100-47-0 C7H5N 103.123 氯化苄 benzyl chloride 100-44-7 C7H7Cl 126.586 乙基苯 ethylbenzene 100-41-4 C8H10 106.167 溴甲苯 benzyl bromide 100-39-0 C7H7Br 171.037 苯乙烯 styrene 100-42-5 C8H8 104.152 乙基-1-13C-苯 ethylbenzene 7-13C 287399-32-0 C8H10 107.156 苯乙炔 phenylacetylene 536-74-3 C8H6 102.136 —— α-Tritiotoluol 6683-73-4 C7H8 94.1326 —— deuteriomethyl-benzene 1861-00-3 C7H8 93.1326 对氯甲苯 4-chlorotoluene 106-43-4 C7H7Cl 126.586 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 对二甲苯 para-xylene 106-42-3 C8H10 106.167 间二甲苯 m-xylene 108-38-3 C8H10 106.167 均三甲苯 1,3,5-trimethyl-benzene 108-67-8 C9H12 120.194 邻二甲苯 o-xylene 95-47-6 C8H10 106.167 苯甲醛 benzaldehyde 100-52-7 C7H6O 106.124 苄胺 benzylamine 100-46-9 C7H9N 107.155 苯甲醇 benzyl alcohol 100-51-6 C7H8O 108.14 氟甲苯 benzyl fluoride 350-50-5 C7H7F 110.131 苄硫醇 phenylmethanethiol 100-53-8 C7H8S 124.207 苄基碘 iodomethylbenzene 620-05-3 C7H7I 218.037 苯甲醛-18O [18O]-benzaldehyde 55076-26-1 C7H6O 108.125 —— 18O-benzyl alcohol 42181-97-5 C7H8O 110.141 乙基苯 ethylbenzene 100-41-4 C8H10 106.167 苯乙烯 styrene 100-42-5 C8H8 104.152 氯化苄 benzyl chloride 100-44-7 C7H7Cl 126.586 苯甲腈 benzonitrile 100-47-0 C7H5N 103.123 —— deuteriomethyl-benzene 1861-00-3 C7H8 93.1326 溴甲苯 benzyl bromide 100-39-0 C7H7Br 171.037 苯乙炔 phenylacetylene 536-74-3 C8H6 102.136 —— α-Tritiotoluol 6683-73-4 C7H8 94.1326 —— [β-11C]Styrene 103731-96-0 C8H8 103.141 对氯甲苯 4-chlorotoluene 106-43-4 C7H7Cl 126.586 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:Fischer,G., Justus Liebigs Annalen der Chemie, 1863, vol. 127, p. 145摘要:DOI:
-
作为产物:描述:参考文献:名称:苄基苯基醚在水相和非极性相中的催化裂解机理摘要:探索了使用镍基和沸石基催化剂在液相中裂解苄基苯基醚(BPE)中的醚键的催化途径。在不存在催化剂的情况下,通过水解在水中选择性裂解C-O键,形成苯酚和苯甲醇作为中间体,然后进行烷基化。通过在523 K下水的离解提供催化反应的水合氢离子。添加HZSM-5后,相对于质子浓度,水解和烷基化速率显着提高。在Ni / SiO 2存在下,选择性氢解作用主要用于裂解C脂族-O键。在双功能Ni / HZSM-5的催化下,氢解反应是主要途径,而不是水解(次要途径)。在非极性十一烷中,非催化热解路线占主导地位。在Ni / SiO 2存在下十一烷中BPE的氢解作用似乎是主要的反应途径或Ni / HZSM-5,几乎完全抑制了自由基反应。密度泛函理论(DFT)的计算有力地支持了BPE在水相和非极性相中提出的C-O键断裂机理。这些计算表明,BPE首先被质子化,随后在水相中水解。DFT计算表明,在非极性溶剂中的自由基反应DOI:10.1016/j.jcat.2013.10.024
-
作为试剂:描述:参考文献:名称:Marckwald, Chemische Berichte, 1892, vol. 25, p. 3106,3107摘要:DOI:
文献信息
-
Iron-catalyzed thioesterification of methylarenes with thiols in water作者:Liang Wang、Jing Cao、Qun Chen、Ming-yang HeDOI:10.1016/j.tetlet.2014.10.155日期:2014.12An iron-catalyzed coupling reaction of methylarenes with thiols leading to thioesters has been developed. The reactions were carried out in water with tert-butyl hydroperoxide (TBHP) as the oxidant and polyoxyethanyl α-tocopheryl sebacate (PTS) as the surfactant. The reaction medium is compatible with a series of functional groups and can be reused.
-
Facile Approach for C(sp3)–H Bond Thioetherification of Isochroman作者:Chun Cai、Jie Feng、Guoping Lu、Meifang LvDOI:10.1055/s-0034-1380125日期:——An unprecedented C–S formation protocol via the direct oxidative C(sp 3 )–H bond thioesterification of isochroman under metal-free conditions was developed. A series of isochroman derivatives could be afforded efficiently by the green, simple, and atom-economical method.
-
Copper-Catalyzed Protodecarboxylation of Aromatic Carboxylic Acids作者:Lukas J. Gooßen、Werner R. Thiel、Nuria Rodríguez、Christophe Linder、Bettina MelzerDOI:10.1002/adsc.200700223日期:2007.10.8A catalyst generated from copper(I) oxide and 4,7-diphenyl-1,10-phenanthroline for the first time allows the catalytic protodecarboxylation even of deactivated aromatic carboxylic acids, giving rise to the corresponding arenes. Based on DFT calculations, a reaction pathway is proposed that accurately reflects the experimental results, such as the observed reactivity order of the substrates.
-
Metal-Free Intermolecular Oxidative C–N Bond Formation via Tandem C–H and N–H Bond Functionalization作者:Abhishek A. Kantak、Shathaverdhan Potavathri、Rose A. Barham、Kaitlyn M. Romano、Brenton DeBoefDOI:10.1021/ja2087085日期:2011.12.14development of a novel intermolecular oxidative amination reaction, a synthetic transformation that involves the simultaneous functionalization of both a N-H and C-H bond, is described. The process, which is mediated by an I(III) oxidant and contains no metal catalysts, provides a rapid and green method for synthesizing protected anilines from simple arenes and phthalimide. Mechanistic investigations
-
Homogeneous Catalytic Hydrogenation of Amides to Amines作者:Jacorien Coetzee、Deborah L. Dodds、Jürgen Klankermayer、Sandra Brosinski、Walter Leitner、Alexandra M. Z. Slawin、David J. Cole-HamiltonDOI:10.1002/chem.201204270日期:2013.8.12Hydrogenation of amides in the presence of [Ru(acac)3] (acacH=2,4‐pentanedione), triphos [1,1,1‐tris‐ (diphenylphosphinomethyl)ethane] and methanesulfonic acid (MSA) produces secondary and tertiary amines with selectivities as high as 93 % provided that there is at least one aromatic ring on N. The system is also active for the synthesis of primary amines. In an attempt to probe the role of MSA and在[Ru(acac)3 ](acacH = 2,4-戊二酮),三[[1,1,1-三(二苯基膦甲基)乙烷]]和甲磺酸(MSA)的存在下进行酰胺加氢生成仲胺和叔胺如果在N上至少有一个芳环,则其选择性高达93%。该系统对伯胺的合成也具有活性。为了探索MSA的作用和反应机理,已经从[Ru(acac)3 ],三醇和MSA或[RuX(OAc)(triphos)]的反应中制备了一系列甲磺酸钠络合物。 (X = H或OAc)或[RuH 2(CO)(triphos )]与MSA。晶体学表征复合物包括:[茹(OAC-κ 1 O)2(H 2O)(triphos)],[Ru(OAc‐κ 2 O,O')(CH 3 SO 3 ‐κ 1 O)(triphos )],[Ru(CH 3 SO 3‐ κ 1 O)2(H 2 O)(三膦)]和[孺2(μ-CH 3 SO 3)3(三磷酸)2 ] [CH 3 SO 3 ],而其他复合物,例如[茹(OAC-κ
表征谱图
-
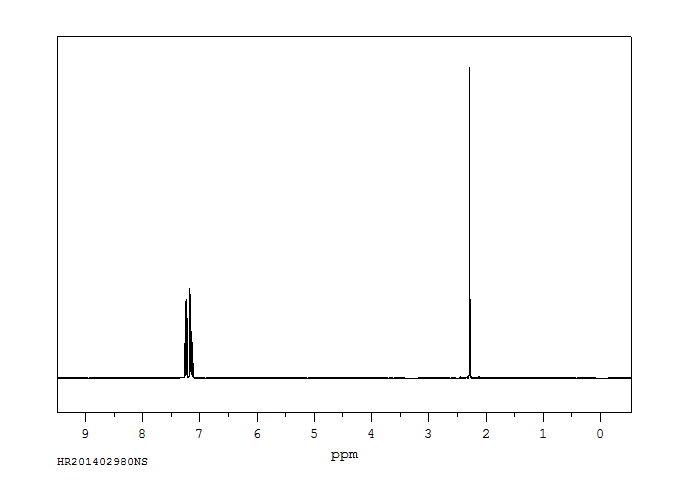
氢谱1HNMR
-
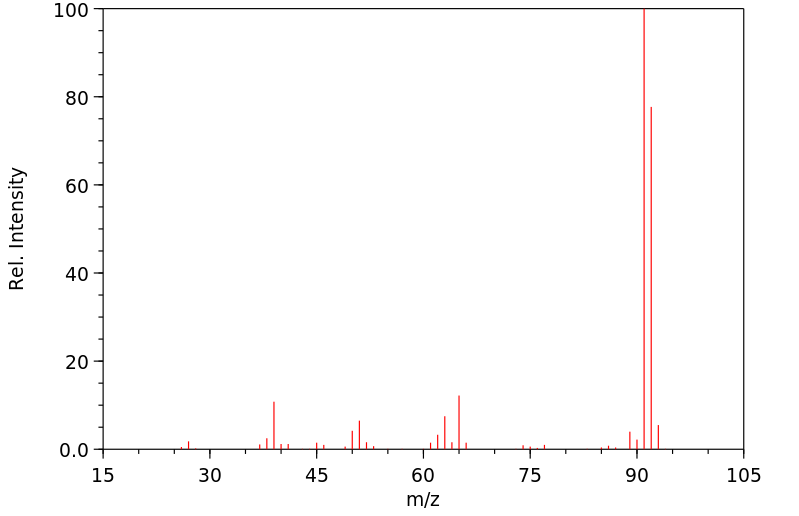
质谱MS
-
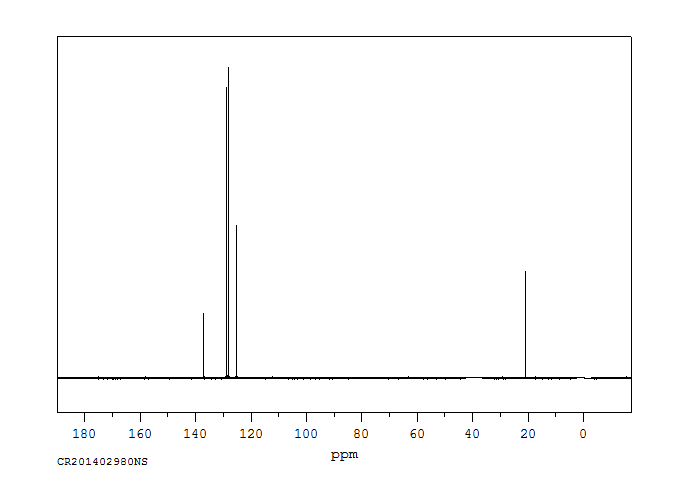
碳谱13CNMR
-
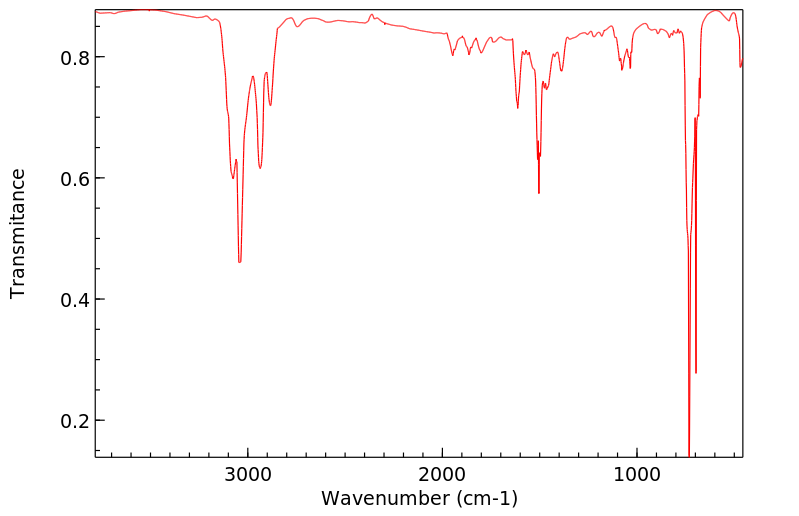
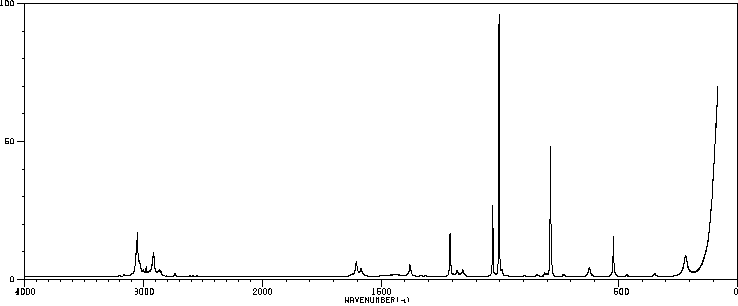
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息