3-Hydroxy-6,8-dimethoxy-1-methyl-naphthalin-2-carbonsaeure-ethylester | 17276-11-8
中文名称
——
中文别名
——
英文名称
3-Hydroxy-6,8-dimethoxy-1-methyl-naphthalin-2-carbonsaeure-ethylester
英文别名
Ethyl 3-hydroxy-6,8-dimethoxy-1-methyl-2-naphthoate;ethyl 3-hydroxy-6,8-dimethoxy-1-methylnaphthalene-2-carboxylate
CAS
17276-11-8
化学式
C16H18O5
mdl
——
分子量
290.316
InChiKey
MSNMOZQVYDYKGW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:21
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.31
-
拓扑面积:65
-
氢给体数:1
-
氢受体数:5
反应信息
-
作为反应物:描述:3-Hydroxy-6,8-dimethoxy-1-methyl-naphthalin-2-carbonsaeure-ethylester 、 甲基锂 以 四氢呋喃 、 乙醚 为溶剂, 反应 1.17h, 以92%的产率得到1-(3-hydroxy-6,8-dimethoxy-1-methylnaphthalen-2-yl)ethanone参考文献:名称:Biomimetic Asymmetric Synthesis of (R)-GTRI-02 and (3S,4R)-3,4-Dihydroxy-3,4-dihydronaphthalen-1(2H)-ones摘要:The NADPH-dependent tetrahydroxynaphthalene reductase (T4HNR) from Magnaporthe grisea was used for the biomimetic synthesis of (R)-GTRI-02 by stereoselective reduction of 1-(3,6,8-trihydroxy-1-methylnaphthalen-2-yl)ethanone. This also led to the isolation of a (3S,4R)-cis-ketodiol formed by T4HNR-catalyzed reduction of the corresponding hydroxynaphthoquinone. Flaviolin and lawsone also reduced to corresponding cis-ketodiols in good yields.DOI:10.1021/ol301305p
-
作为产物:描述:2-(3,5-dimethoxyphenyl)acetyl chloride 在 甲烷磺酸 、 四磷十氧化物 、 sodium hydride 作用下, 以 四氢呋喃 为溶剂, 反应 2.0h, 生成 3-Hydroxy-6,8-dimethoxy-1-methyl-naphthalin-2-carbonsaeure-ethylester参考文献:名称:Biomimetic Asymmetric Synthesis of (R)-GTRI-02 and (3S,4R)-3,4-Dihydroxy-3,4-dihydronaphthalen-1(2H)-ones摘要:The NADPH-dependent tetrahydroxynaphthalene reductase (T4HNR) from Magnaporthe grisea was used for the biomimetic synthesis of (R)-GTRI-02 by stereoselective reduction of 1-(3,6,8-trihydroxy-1-methylnaphthalen-2-yl)ethanone. This also led to the isolation of a (3S,4R)-cis-ketodiol formed by T4HNR-catalyzed reduction of the corresponding hydroxynaphthoquinone. Flaviolin and lawsone also reduced to corresponding cis-ketodiols in good yields.DOI:10.1021/ol301305p
文献信息
-
Metabolic Products of Fungi. XXVI. Synthesis of racemic Ustilaginoidin A and Its Related Compounds. (1). Synthesis of 2.2', 4.4', 5.5', 7.7'-Octamethoxy-1.1'-binaphthalene (=Product A Octamethyl Ether).作者:Eisaku Morishita、Shoji ShibataDOI:10.1248/cpb.15.1765日期:——Octamethyl ether of product A which was derived from ustilaginoidin A by alkaline degradation was synthesized by the Ullmann condensation of dimethyl ether of 5-bromo-6, 8-dimethyl-1, 3-naphthalenediol (XXI). Starting from 3, 5-dimethoxyphenylacetic acid (IX), the 2-bromo derivative (VII) was prepared which was C-acetylated to afford XX, and then cyclized to form a bromonaphthalene derivative (XXI), whose methyl ether is XXII. The position of C-C linkage connecting two monomeric moieties of ustilaginoidins has unequivocally been established. Rearrangement of bromine atom during the process of methylation of bromohydroxynaphthalenes and cyclization forming the naphthalene nucleus were also discussed.
表征谱图
-
氢谱1HNMR
-
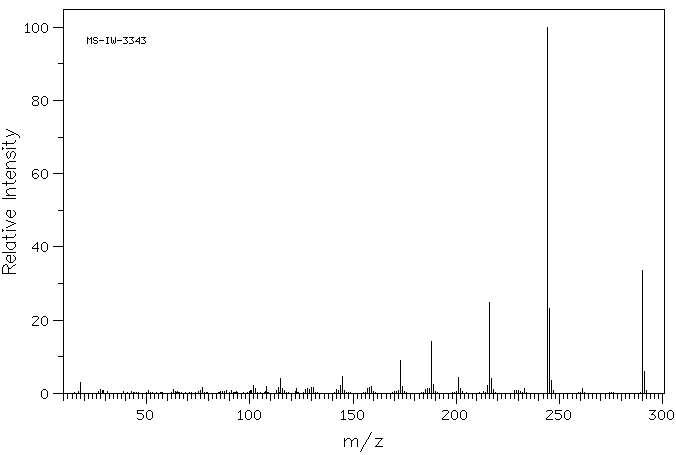
质谱MS
-
碳谱13CNMR
-
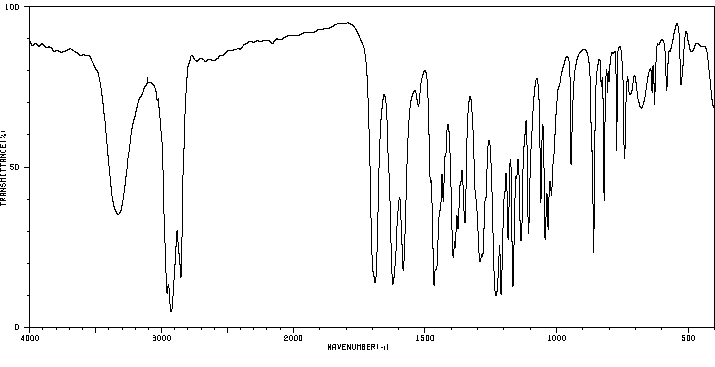
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮