2,5-dimethyl-piperazine | 1119702-25-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:24.1
-
氢给体数:2
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,5-二甲基哌嗪 2,5-Dimethylpiperazin 106-55-8 C6H14N2 114.191 —— cis-2,5-dimethylpiperazine 6284-84-0 C6H14N2 114.191 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— cis-2,5-dimethylpiperazine 6284-84-0 C6H14N2 114.191 (2R,5S)-1,2,5-三甲基哌嗪 N-Methyl-trans-2,5-dimethyl-piperazin 1046788-78-6 C7H16N2 128.217 —— (+/-)-1,2r,4,5t-tetramethyl-piperazine 697728-90-8 C8H18N2 142.244
反应信息
-
作为反应物:描述:2,5-dimethyl-piperazine 在 potassium carbonate 作用下, 以 乙醇 、 乙腈 为溶剂, 反应 72.0h, 生成 (+)-4-[(ΑR)-Α-((2S,5R)-4-烯丙基-2,5-二甲基-1-哌嗪基)-3-甲氧基苄基]-N,N-二乙基苯酰胺参考文献:名称:麻醉受体介导现象的探针。23.高选择性δ激动剂(+)-4-[(αR)-α-((2S,5R)-4-烯丙基-2,5-二甲基-1-哌嗪基]的合成,阿片受体结合和生物测定)-3-甲氧基苄基] -N,N-二乙基苯甲酰胺(SNC 80)和相关的新型非肽δ阿片受体配体。摘要:高选择性δ(δ)阿片受体激动剂SNC 80 [(+)-4-[[αR)-α-((2S,5R)-4-烯丙基-2,5-二甲基-1-哌嗪基)-3 -甲氧基苄基] -N,N-二乙基苯甲酰胺,(+)-21]和新颖的光学纯衍生物是由1-烯丙基-反式-2,5-二甲基哌嗪的对映体合成的(2)。合成了哌嗪(+/-)-2,并且通过外消旋物与樟脑酸的旋光拆分,以大于99%的光学纯度以克数获得了其对映异构体。通过用(+)-樟脑酸对盐进行X射线分析,确定(+)-2的绝对构型为2S,5R。由于已知原料的手性,并且通过单晶X射线分析获得了化合物(-)-21,(-)-22和(+)-23的相对构型,可以确定整个系列的绝对立体化学。大鼠脑制剂中的放射性受体结合研究表明,甲基醚(+)-21(SNC 80)和(-)-25对大鼠δ受体具有很强的选择性,对δ受体的纳摩尔亲和力低,而对鼠mu(mu)仅微摩尔的亲和力阿片受体。化合物(-)DOI:10.1021/jm960319n
-
作为产物:描述:参考文献:名称:Method of manufacturing cis 2, 5-dimethyl-piperazine摘要:该规范声称通过以下过程:(1) 将顺式或反式-2,5-二甲基哌嗪异构化,与含镍的加氢-脱氢催化剂在氢气气氛中(最好是在压力下)加热,以产生两者的混合物;以及(2) 通过将异丙醇胺加热至180-260摄氏度,在至少200磅/平方英寸的氢气压力下,在至少2小时的时间内,在镍加氢-脱氢催化剂存在下,生产至少含有50%反式-2,5-二甲基哌嗪的混合物,其中还包含一些顺式-2,5-二甲基哌嗪和2,5-二甲基吡啶。在这些条件下获得的平衡混合物约含有80%的反式化合物,因此如果初始情况下反式化合物的含量低于80%,则可以使用过程(1)来富集反式形式,或者如果顺式形式的含量低于10%,则可以富集顺式形式。类似地,通过将顺式形式加入异丙醇胺,可以增加由过程(2)产生的反式化合物的量。过程(2)的优选条件为温度为200-240摄氏度(例如220-240摄氏度),氢气压力为800-1200磅/平方英寸,加热4小时或更长时间,以及1.25克或更多(例如2.5克)的Raney镍型合金骨架催化剂。提供了在不同实验条件下产量和转化率变化的信息。将经过过滤的反应产物加热以蒸馏水和2,5-二甲基吡啶,并将未反应的异丙醇胺与乙苯或二甲苯组成共沸物一起去除;这些产品可以回收利用。顺式和反式-2,5-二甲基哌嗪可以通过分馏部分分离,反式形式可以通过从熔融物中结晶纯化,而顺式形式可以通过在庚烷中溶解来纯化。这种分离可以连续或分批进行,适用于前者的合适装置已有示例。参考规范791,050。公开号:US03067201A1
-
作为试剂:描述:溴代硝基甲烷 、 3-(3-chlorophenyl)-1-phenylprop-2-en-1-one 在 三氟乙酸 、 奎宁胺 、 2,5-dimethyl-piperazine 作用下, 以 氯仿 、 二氯甲烷 为溶剂, 反应 24.0h, 以75%的产率得到1-(2-(3-chlorophenyl)-3-nitrocyclopropyl)-1-phenylmethanone参考文献:名称:通过有机催化共轭溴硝基烷烃向α,β-不饱和烯酮的对映选择性合成功能化的硝基环丙烷摘要:提出了通过将各种溴硝基烷烃有机共轭加成到α,β-不饱和烯酮体系中来官能化硝基环丙烷的一般对映选择性合成方法。该过程可通过9-氨基-9-脱氧表奎宁1 d的盐有效催化,是制备具有高对映异构和非对映异构选择性的合成和生物学上重要的环丙烷的有力方法。由于仅使用0.6当量的溴硝基甲烷作为试剂,通过使用手性1 d作为其动力学拆分的高效催化剂,对映体纯(S)-2 e(97% ee在51%转化率下,选择性s = 120) 。DOI:10.1002/chem.200801651
文献信息
-
Dual Pharmacophores - PDE4-Muscarinic Antagonistics申请人:Callahan James Francis公开号:US20090197871A1公开(公告)日:2009-08-06The present invention is directed to novel compounds of Formula (I), pharmaceutical compositions and their use in therapy, for example as inhibitors of phosphodiesterase type IV (PDE4) and as antagonists of muscarinic acetylcholine receptors (mAChRs), in the treatment of/and or prophylaxis of respiratory diseases, including antiinflammatory and/or allergic diseases such as chronic obstructive pulmonary disease (COPD), asthma, rhinitis (e.g. allergic rhinitis), atopic dermatitis or psoriasis.
-
Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives作者:Ebtehal Al-Abdullah、Hanaa Al-Tuwaijri、Hanan Hassan、Monirah Al-Alshaikh、Elsayed Habib、Ali El-EmamDOI:10.3390/molecules20058125日期:——The reaction of 1-adamantyl isothiocyanate 4 with the various cyclic secondary amines yielded the corresponding N-(1-adamantyl)carbothioamides 5a–e, 6, 7, 8a–c and 9. Similarly, the reaction of 4 with piperazine and trans-2,5-dimethylpiperazine in 2:1 molar ratio yielded the corresponding N,N'-bis(1-adamantyl)piperazine-1,4-dicarbothioamides 10a and 10b, respectively. The reaction of N-(1-adamantyl)-4-ethoxycarbonylpiperidine-1-carbothioamide 8c with excess hydrazine hydrate yielded the target carbohydrazide 11, in addition to 4-(1-adamantyl)thiosemicarbazide 12 as a minor product. The reaction of the carbohydrazide 11 with methyl or phenyl isothiocyanate followed by heating in aqueous sodium hydroxide yielded the 1,2,4-triazole analogues 14a and 14b. The reaction of the carbohydrazide 11 with various aromatic aldehydes yielded the corresponding N'-arylideneamino derivatives 15a–g. The compounds 5a–e, 6, 7, 8a–c, 9, 10a, 10b, 14a, 14b and 15a–g were tested for in vitro antimicrobial activity against certain strains of pathogenic Gram-positive and Gram-negative bacteria and the yeast-like fungus Candida albicans. The compounds 5c, 5d, 5e, 6, 7, 10a, 10b, 15a, 15f and 15g showed potent antibacterial activity against one or more of the tested microorganisms. The oral hypoglycemic activity of compounds 5c, 6, 8b, 9, 14a and 15b was determined in streptozotocin (STZ)-induced diabetic rats. Compound 5c produced significant reduction of serum glucose levels, compared to gliclazide.1-金刚烷基异硫氰酸酯4与各种环状仲胺反应,生成了相应的N-(1-金刚烷基)硫代碳酰胺5a–e、6、7、8a–c和9。类似地,4与哌嗪和反式-2,5-二甲基哌嗪在2:1摩尔比下反应,分别生成了相应的N,N'-双(1-金刚烷基)哌嗪-1,4-二硫代碳酰胺10a和10b。N-(1-金刚烷基)-4-乙氧羰基哌啶-1-硫代碳酰胺8c与过量水合肼反应,除了生成目标羧酰肼11外,还生成了少量的4-(1-金刚烷基)缩氨基硫脲12。羧酰肼11与甲基或苯基异硫氰酸酯反应后在氢氧化钠水溶液中加热,生成了1,2,4-三唑类似物14a和14b。羧酰肼11与各种芳香醛反应,生成了相应的N'-芳亚胺氨基衍生物15a–g。化合物5a–e、6、7、8a–c、9、10a、10b、14a、14b和15a–g对某些致病性革兰氏阳性菌、革兰氏阴性菌和类酵母菌白念珠菌进行了体外抗微生物活性测试。化合物5c、5d、5e、6、7、10a、10b、15a、15f和15g对一种或多种测试的微生物显示出强效的抗菌活性。化合物5c、6、8b、9、14a和15b对链脲佐菌素(STZ)诱导的糖尿病大鼠进行了口服降糖活性测定。化合物5c显著降低了血清葡萄糖水平,相比于格列齐特。
-
Triazene derivatives of (1,<i>x</i>)-diazacycloalkanes. Part X. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]-<i>trans</i>-2,5-dimethylpiperazines作者:Vanessa Renee Little、Reid Tingley、Keith VaughanDOI:10.1139/cjc-2014-0107日期:2014.7
A series of 1,4-di[2-aryl-1-diazenyl]-trans-2,5-dimethylpiperazines(5a–5m), have been synthesized by the reaction of trans-2,5-dimethylpiperazine with two equivalents of the appropriate diazonium salt. The products have been characterized by IR and NMR spectroscopy, and the molecular composition has been verified by high-resolution EI mass spectrometry with accurate mass measurement of the molecular ion. The presence of stereocenters at C2 and C5 of the piperazine ring in the bis-triazene (5) creates two unique pairs of diastereotopic protons in the methylene groups at positions 3 and 6 of the piperazine ring, as evidenced by the complexity of the NMR spectra, which nevertheless can be fully assigned in some cases, such as the anisyl- (5i) and phenyl- (5j) derivatives. The assignment of the proton and carbon signals in the tolyl- derivative (5h) has been aided by the use of 2D NMR HSQC spectroscopy. These results compare favorably with assignments of proton and carbon signals reported previously for triazenes of type 1 and bis-triazenes of types 3 and 4b.
一系列1,4-二[2-芳基-1-重氮基]-反式-2,5-二甲基哌嗪(5a-5m)已通过反应反式-2,5-二甲基哌嗪与适当重氮盐的两当量合成。 通过红外和核磁共振光谱对产物进行了表征,并通过高分辨率EI质谱进行了分子离子的准确质量测定来验证分子组成。 在双三重偶氮基(5)的哌嗪环的C2和C5处存在立体中心,形成了在哌嗪环的3和6位置的亚稳位甲基基团中的两对独特的非等价质子,这一事实可通过核磁共振光谱的复杂性来证明,尽管在某些情况下可以完全指定,例如苯基(5i)和苯基(5j)衍生物。 通过使用2D核磁共振HSQC光谱,对甲苯衍生物(5h)中的质子和碳信号的指定得到了帮助。 这些结果与先前报告的类型1三重偶氮基和类型3和4b双三重偶氮基的质子和碳信号的指定相比较有利。 -
Piperazine and homopiperazine compounds
-
[EN] SUBSTITUTED PIPERAZINES, (1,4) DIASZEPINES, AND 2,5-DIAZABICYCLO (2.2.1) HEPTANES AS HISTAMINE H1 AND/OR H3 ANTAGONISTS OR HISTAMINE H3 REVERSE ANTAGONISTS<br/>[FR] PIPERAZINES, (1,4) DIAZEPINES, ET 2,5-DIAZABICYCLO (2.2.1) HEPTANES SUBSTITUES EN TANT QU'ANTAGONISTES DE L'HISTAMINE H1 ET/OU H3 OU ANTAGONISTES INVERSES DE L'HISTAMINE H3申请人:GLAXO GROUP LTD公开号:WO2004035556A1公开(公告)日:2004-04-29The present invention relates to novel piperazine and azepine derivatives having pharmacological activity, processes for their preparation, to compositions containing them and to their use in the treatment of neurodegenerative disorders including Alzheimer's disease.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
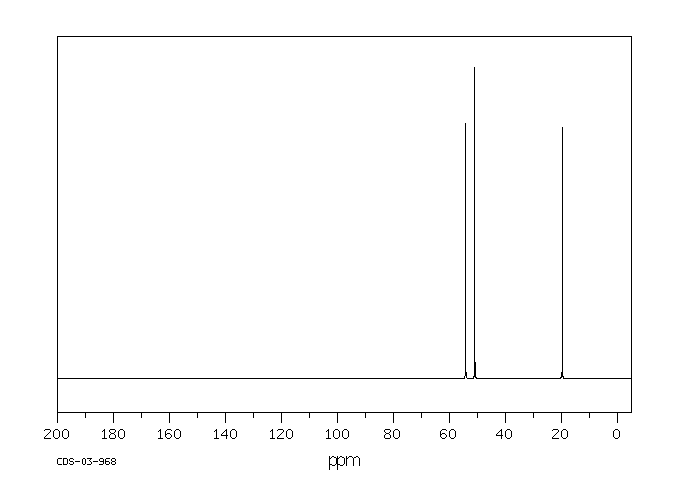
碳谱13CNMR
-
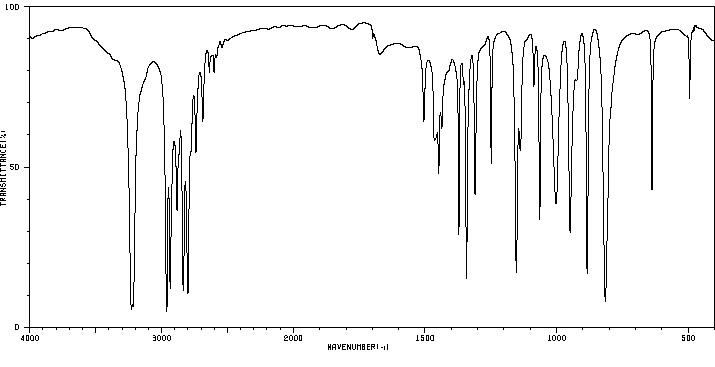
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息